Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Lanthanum to lower phosphate in rocks outside of aquarium
- Thread starter kenchilada
- Start date
- Tagged users None
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
About lanthanum chloride use.
IMO, the primary unknown is the reason some see fish toxicity and most do not.
Are there other things about it that you believe reflect uncertainty?
The fundamental chemistry processes are quite straightforward.
Now the 250G tank is left reading 10ppm phosphate. There is no more aragonite sink left in the tank, so I should be able to easily remove it. May try a 200G water change?
IMO, the primary unknown is the reason some see fish toxicity and most do not.
Totally agree. I was considering putting my sailfin tang in quarantine with clean saltwater and adding the lanthanum product as a test but chickened out.
My hunch is it’s not the precipitate, but that’s based on my own worthless anecdotes.
I've only used Brightwell Phosphat-E, but here are some things...Are there other things about it that you believe reflect uncertainty?
The fundamental chemistry processes are quite straightforward.
Dosing into a sock seems of questionable value. It certainly does nothing for my fish. I suppose if you keep some portion of the precipitate out of the tank there is value in that (less test interference?), but people portray it as a solution to fish deaths. Many that use a sock and do not lose a fish report success and then post it as advice when most simply had no susceptible fish.
Further there is much uncertainty around micron size of mechanical filtration. An old post from Reef Central mentions "scientific experiments show 0.45 micron filters work the best", yet it is common to see everything from 1-25 micron socks recommended (usually attempting to benefit the fish).
I also think the conventional way we dose this into socks causes most LaCl product to simply flow out of the sock unreacted. I have seen posts state that most LaCl will react within 90 seconds.
I have seen no benefit from dillution and slow dripping into 1 micron socks versus adding product directly to the aquarium. The Brightwell instructions just say "Add product to aquarium near a mechanical filter or protein skimmer intake". It seems just as irritable to my fish and just as effective at removing PO4 regardless.
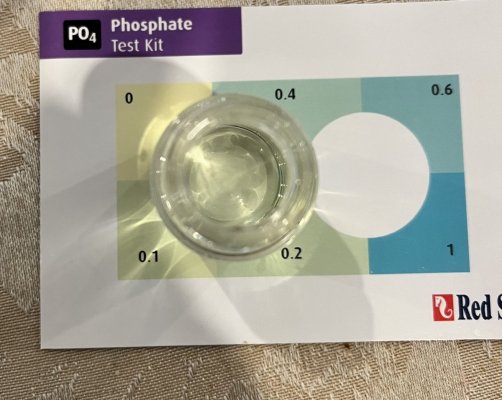
Poor test resolution at high range adds confusion around what is happening. Trying to interpret from 0-17PPM using six possible colors is not ideal. Plus lanthanum phosphate in the sample further confuses things.
It is commonly posted that LaCl is only effective to reduce phosphate to around 0.1-0.2ppm, which I can confirm in my usage as well. I have not found an explanation for why this happens but I would love to know why!
I have more thoughts but this is already long enough. For anyone reading, the above is anecdotes from my own usage and should not be construed as useful advice. I'm curious to understand it more, but it is a miracle product for my scenario and thankful to have it.
I've read somewhere that when the phosphate level is lower, the lanthanum turns into lanthanum carbonate instead of lanthanum phosphate. So instead of reducing phosphate its more likely to reduce alkalinity.I have not found an explanation for why this happens but I would love to know why!
Whether or not phosphate is still likely to bind to lanthanum carbonate, I don't know.
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
I've read somewhere that when the phosphate level is lower, the lanthanum turns into lanthanum carbonate instead of lanthanum phosphate. So instead of reducing phosphate its more likely to reduce alkalinity.
Whether or not phosphate is still likely to bind to lanthanum carbonate, I don't know.
That is likely, IMO.
Problem solved. I am dosing beyond manufacturers recommended strength and see zero effects on my sailfin tang.
100mL Brightwell Phosphat-E undiluted over 24 hours on my 250G system.

Thanks @Garf for your input
100mL Brightwell Phosphat-E undiluted over 24 hours on my 250G system.
Thanks @Garf for your input
Similar threads
- Replies
- 18
- Views
- 408
- Replies
- 1
- Views
- 373
- Replies
- 17
- Views
- 564
- Replies
- 43
- Views
- 1,506
- Replies
- 16
- Views
- 345