- Joined
- Sep 21, 2018
- Messages
- 6,958
- Reaction score
- 7,415
One year ago I started carbon dosing my fish only aquarium. There were no issues in the four year old aquarium that carbon dosing might address. I just wanted to learn more about the procedure that uses carbon to increase bacteria growth, and possibly, develop a simple test that might assist in selecting the daily dose size. My plan was to start at a low dose and spend the year increasing the dose and monitoring aquarium parameters. Nitrate, phosphate, alkalinity, calcium, organic carbon level, skimmer performance, and Caulerpa production were measured weekly. Oxygen and pH were monitored in a few small scale experiments
Calcium acetate was my choice for a carbon source. I read that ethanol might stimulate cyanobacteria growth, and as far as I could determine, acetic acid work just as well. I didn’t like adding acid to a bicarbonate buffer system and decided to mix solid kalkwasser with vinegar to make a basic solution of calcium acetate.
Observations became a bit confounded towards the end of the year. I decided the fish needed more room and the resulting system changes complicated the comparison of early results with those near the end of year. The changes included connecting a 75 gallon tank to the existing 40 gallon tank with an illuminated 10 gallon sump. Then the rocks and half the old substrate were moved to the new tank. New substrate was added and the fish were moved. A new 10 gallon dark sump was added and then the 40 gallon tank was removed from the system. Charts are marked when the 40 gallon tank was disconnected. Below is a summary of my observations on carbon dosing.
Effect on Alkalinity
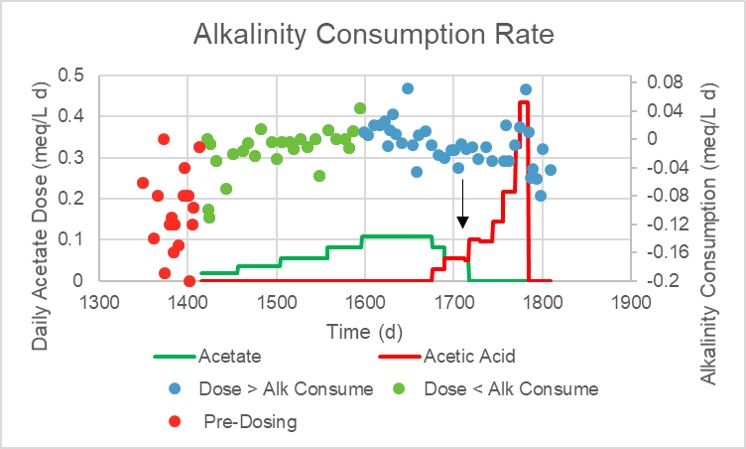
I started with a low daily dose of calcium acetate, 0.08 mL/gallon, followed by days of observation before increasing it slightly. The “Alkalinity Consumption Rate” chart shows how daily doses progressed in size and the effect it had on alkalinity consumption (expressed as a negative number). Average daily alkalinity consumption for my system before dosing was 0.1 meq/L and quite variable. Once dosing started, the variability seemed to suddenly decrease. Because acetate when metabolized produces one bicarbonate, the average daily alkalinity consumption slowly decreased. When the daily dose of acetate reached 0.1 meq/L, the alkalinity consumption rate became positive and the alkalinity began to increase.
To demonstrate that the alkalinity effect was a result of the acetate alkalinity, at day 1680 the dose was changed in steps from 100% acetate to 100% acetic acid while keeping the total amount of the carbon added per day constant. The average daily alkalinity consumption decreased to about half the pre-dosing levels and with seemingly less variability. At the point in time marked with an arrow, the old 40 gallon tank was removed.
Dosing was then increased more quickly because of a rapid increase in nitrate level, likely a result of disconnecting the 40 gallon tank. The maximum daily dose achieved was 2 mL/gallon per day. Within days of reaching this dose and reducing the nitrate level, dosing was abruptly stopped.
Effect on Nitrate.
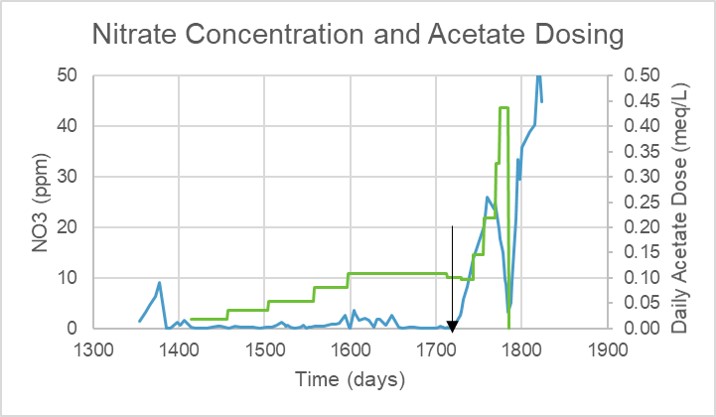
As stated above, my system was not experiencing elevated levels of nitrate and phosphate (see “Nitrate Concentration and Acetate Dosing” chart). Not until a sharp rise in nitrate concentration around day 1720 (see arrow) could acetate induced nitrate consumption be observed.
In the mean time, a series of small scale experiments were performed with the objective of determining the consumption rates for nitrate, acetate and phosphate. In a typical experiment, calcium acetate was added to a sample of aquarium water to give an acetate concentration of 4 mM. This corresponded to a single 19 mL dose of vinegar per gallon of aquarium water. When the aquarium water nitrate concentration was too low for experiments, the sample was spiked with a solution of sodium nitrate. In early experiments, phosphate was discovered to increase nitrate consumption, decreasing the time for elimination of nitrate from about a month to within a week. Additional studies with increasing amounts of phosphate demonstrated a substantial positive impact on the nitrate consumption rate. Dosed iron did not seem to change the nitrate consumption rate.
A trend that proved consistent throughout this study was the large amount of acetate consumed compared to that of nitrate (see “Acetate and Nitrate Consumption Rates” chart). In some experiments, acetate was consumed with little or no nitrate reduction. As a reference point, when acetate is being consumed solely for anaerobic reduction of nitrate to nitrogen gas, the rates of consumption of acetate and nitrate are nearly the same.

Similar to small experiment acetate consumption rates, a large amount of acetate was dosed to the aquarium to consume nitrate (see “Acetic Acid Needed to Consume Nitrate” chart). Acetate dosed to the aquarium to reduce the nitrate at day 1760 was 8 mM, compared to the corresponding nitrate reduction of 0.37 mM. During this time, 0.0009 mM of phosphate was consumed. Nutrient ratios for this event were C:N 43 and N 434.
434.

Effect on Caulerpa Harvest.
Caulerpa growing in a ten gallon refugium is used to help control nitrate and phosphate levels. Did carbon dosing have a measurable impact on Caulerpa productivity? The “Caulerpa Harvest” plot shows the running total of the Caulerpa harvest through the dosing period. Except for a slow down about half way through the dosing period, acetate dosing did not seem to have an impact. Not shown in this data, however, is the die back of the Caulerpa and increased growth of hair algae in the sump after dosing concluded. The Caulerpa normally cycles through high and low growth periods, but the level of die back this time was complete. Coincidence? New tank issue? Dosing consequence?

Effect on Glass Scraping Frequency
There was little change in how often I cleaned the glass until the daily dose neared 2 mL per gallon. At the top dose, the sides needed cleaning every several days.
The nature of the growth was not slimy but rather a drier material, leading me to initially think think it was a calcium precipitate. Since it didn’t dissolve in acid, I concluded this was bacteria. After the last dose, I did not remove the white film that accumulated, but observed it for a week a period of a week. The white film became thinner within days of ending the daily dose of acetic acid. The clearing process was uneven and splotchy, with definite areas clearing faster than others along with small areas distinctly circular and clear.
Effect on Organic Matter Level
I have been exploring the use of chlorine consumption as an indicator of the level of organic matter in the water as a way to judge when to add fresh GAC. The “Chlorine Consumption by Marine Aquarium Water” chart shows the trend in chlorine consumption during dosing. The red “X’s” indicate the addition of the 75 gallon tank, the 10 gallon dark sump and the removal of the 40 gallon tank, respectively. Two increasing chlorine consumption trends seem to be related to dosing, the rise in chlorine consumption corresponding to the increase in daily dose of vinegar and the large spike after the abrupt cessation of dosing. Because of all the changes to the system during the first trend, the link between rising chlorine demand and increasing daily dose is not conclusive. The situation is a little more straightforward for linking the spike in chlorine demand and the abrupt cessation of dosing. The sudden loss of organic carbon could have led to a bacterial population crash that could not be rapidly cleared by the GAC and skimmer. After about 30 days the chlorine consumption had returned to normal.

Conclusion. I had hoped to gather enough information to develop a simple test for predicting the organic carbon dose that would be needed to reduce a given nitrate level. What I found was that a large amount of acetate is consumed relative to nitrate and that acetate is consumed even during periods of undetectable nitrate consumption. Also, the small scale experiments designed with a single large dose of acetate provided no information about the amount of acetate needed to start the process of nitrate reduction. Is a large initial dose an option for aquariums? This work does not provide an answer, although rarely did the large single dose turn experiment samples turn cloudy. Phosphate turned out to be important to nitrate consumption on small scale and might be similarly important in aquariums.
I am left to ponder whether carbon dosing can begin at 2 mL per gallon per day when dosing vinegar and should it include phosphate when it is low in the aquarium or when there is little progress in nitrate reduction?

Calcium acetate was my choice for a carbon source. I read that ethanol might stimulate cyanobacteria growth, and as far as I could determine, acetic acid work just as well. I didn’t like adding acid to a bicarbonate buffer system and decided to mix solid kalkwasser with vinegar to make a basic solution of calcium acetate.
Observations became a bit confounded towards the end of the year. I decided the fish needed more room and the resulting system changes complicated the comparison of early results with those near the end of year. The changes included connecting a 75 gallon tank to the existing 40 gallon tank with an illuminated 10 gallon sump. Then the rocks and half the old substrate were moved to the new tank. New substrate was added and the fish were moved. A new 10 gallon dark sump was added and then the 40 gallon tank was removed from the system. Charts are marked when the 40 gallon tank was disconnected. Below is a summary of my observations on carbon dosing.
Effect on Alkalinity
I started with a low daily dose of calcium acetate, 0.08 mL/gallon, followed by days of observation before increasing it slightly. The “Alkalinity Consumption Rate” chart shows how daily doses progressed in size and the effect it had on alkalinity consumption (expressed as a negative number). Average daily alkalinity consumption for my system before dosing was 0.1 meq/L and quite variable. Once dosing started, the variability seemed to suddenly decrease. Because acetate when metabolized produces one bicarbonate, the average daily alkalinity consumption slowly decreased. When the daily dose of acetate reached 0.1 meq/L, the alkalinity consumption rate became positive and the alkalinity began to increase.
To demonstrate that the alkalinity effect was a result of the acetate alkalinity, at day 1680 the dose was changed in steps from 100% acetate to 100% acetic acid while keeping the total amount of the carbon added per day constant. The average daily alkalinity consumption decreased to about half the pre-dosing levels and with seemingly less variability. At the point in time marked with an arrow, the old 40 gallon tank was removed.
Dosing was then increased more quickly because of a rapid increase in nitrate level, likely a result of disconnecting the 40 gallon tank. The maximum daily dose achieved was 2 mL/gallon per day. Within days of reaching this dose and reducing the nitrate level, dosing was abruptly stopped.
Effect on Nitrate.
As stated above, my system was not experiencing elevated levels of nitrate and phosphate (see “Nitrate Concentration and Acetate Dosing” chart). Not until a sharp rise in nitrate concentration around day 1720 (see arrow) could acetate induced nitrate consumption be observed.
In the mean time, a series of small scale experiments were performed with the objective of determining the consumption rates for nitrate, acetate and phosphate. In a typical experiment, calcium acetate was added to a sample of aquarium water to give an acetate concentration of 4 mM. This corresponded to a single 19 mL dose of vinegar per gallon of aquarium water. When the aquarium water nitrate concentration was too low for experiments, the sample was spiked with a solution of sodium nitrate. In early experiments, phosphate was discovered to increase nitrate consumption, decreasing the time for elimination of nitrate from about a month to within a week. Additional studies with increasing amounts of phosphate demonstrated a substantial positive impact on the nitrate consumption rate. Dosed iron did not seem to change the nitrate consumption rate.
A trend that proved consistent throughout this study was the large amount of acetate consumed compared to that of nitrate (see “Acetate and Nitrate Consumption Rates” chart). In some experiments, acetate was consumed with little or no nitrate reduction. As a reference point, when acetate is being consumed solely for anaerobic reduction of nitrate to nitrogen gas, the rates of consumption of acetate and nitrate are nearly the same.
Similar to small experiment acetate consumption rates, a large amount of acetate was dosed to the aquarium to consume nitrate (see “Acetic Acid Needed to Consume Nitrate” chart). Acetate dosed to the aquarium to reduce the nitrate at day 1760 was 8 mM, compared to the corresponding nitrate reduction of 0.37 mM. During this time, 0.0009 mM of phosphate was consumed. Nutrient ratios for this event were C:N 43 and N
Effect on Caulerpa Harvest.
Caulerpa growing in a ten gallon refugium is used to help control nitrate and phosphate levels. Did carbon dosing have a measurable impact on Caulerpa productivity? The “Caulerpa Harvest” plot shows the running total of the Caulerpa harvest through the dosing period. Except for a slow down about half way through the dosing period, acetate dosing did not seem to have an impact. Not shown in this data, however, is the die back of the Caulerpa and increased growth of hair algae in the sump after dosing concluded. The Caulerpa normally cycles through high and low growth periods, but the level of die back this time was complete. Coincidence? New tank issue? Dosing consequence?
Effect on Glass Scraping Frequency
There was little change in how often I cleaned the glass until the daily dose neared 2 mL per gallon. At the top dose, the sides needed cleaning every several days.
The nature of the growth was not slimy but rather a drier material, leading me to initially think think it was a calcium precipitate. Since it didn’t dissolve in acid, I concluded this was bacteria. After the last dose, I did not remove the white film that accumulated, but observed it for a week a period of a week. The white film became thinner within days of ending the daily dose of acetic acid. The clearing process was uneven and splotchy, with definite areas clearing faster than others along with small areas distinctly circular and clear.
Effect on Organic Matter Level
I have been exploring the use of chlorine consumption as an indicator of the level of organic matter in the water as a way to judge when to add fresh GAC. The “Chlorine Consumption by Marine Aquarium Water” chart shows the trend in chlorine consumption during dosing. The red “X’s” indicate the addition of the 75 gallon tank, the 10 gallon dark sump and the removal of the 40 gallon tank, respectively. Two increasing chlorine consumption trends seem to be related to dosing, the rise in chlorine consumption corresponding to the increase in daily dose of vinegar and the large spike after the abrupt cessation of dosing. Because of all the changes to the system during the first trend, the link between rising chlorine demand and increasing daily dose is not conclusive. The situation is a little more straightforward for linking the spike in chlorine demand and the abrupt cessation of dosing. The sudden loss of organic carbon could have led to a bacterial population crash that could not be rapidly cleared by the GAC and skimmer. After about 30 days the chlorine consumption had returned to normal.
Conclusion. I had hoped to gather enough information to develop a simple test for predicting the organic carbon dose that would be needed to reduce a given nitrate level. What I found was that a large amount of acetate is consumed relative to nitrate and that acetate is consumed even during periods of undetectable nitrate consumption. Also, the small scale experiments designed with a single large dose of acetate provided no information about the amount of acetate needed to start the process of nitrate reduction. Is a large initial dose an option for aquariums? This work does not provide an answer, although rarely did the large single dose turn experiment samples turn cloudy. Phosphate turned out to be important to nitrate consumption on small scale and might be similarly important in aquariums.
I am left to ponder whether carbon dosing can begin at 2 mL per gallon per day when dosing vinegar and should it include phosphate when it is low in the aquarium or when there is little progress in nitrate reduction?


















