As alkalinity consumption has increased in my tank, it's become increasingly difficult to maintain alkalinity stability, even with Trident controlled dosing. I've noticed that whenever we cook with the range (or oven), indoor Co2 increases. This drives pH down which, in turn, decreases alkalinity consumption. I'm not concerned with the pH swing itself, just the resulting impacts on alkalinity consumption as they pertain to dosing. Trident control is very much a reactive process and it seems to be struggling with the fluctuations.
Below are some graphs of data I've collected:
First, Co2 (right side of graph cut-off to maintain date/time consistency across all plots):
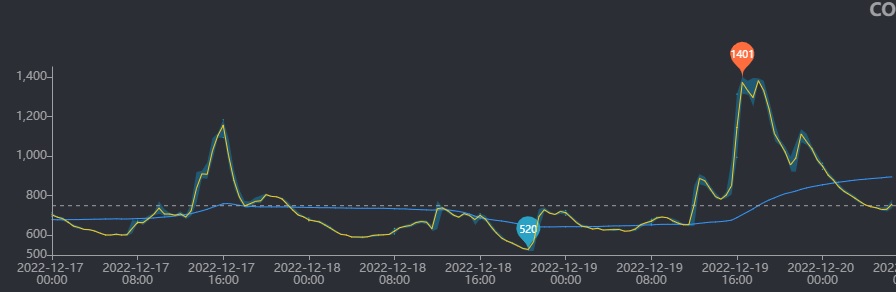
Next, alk:
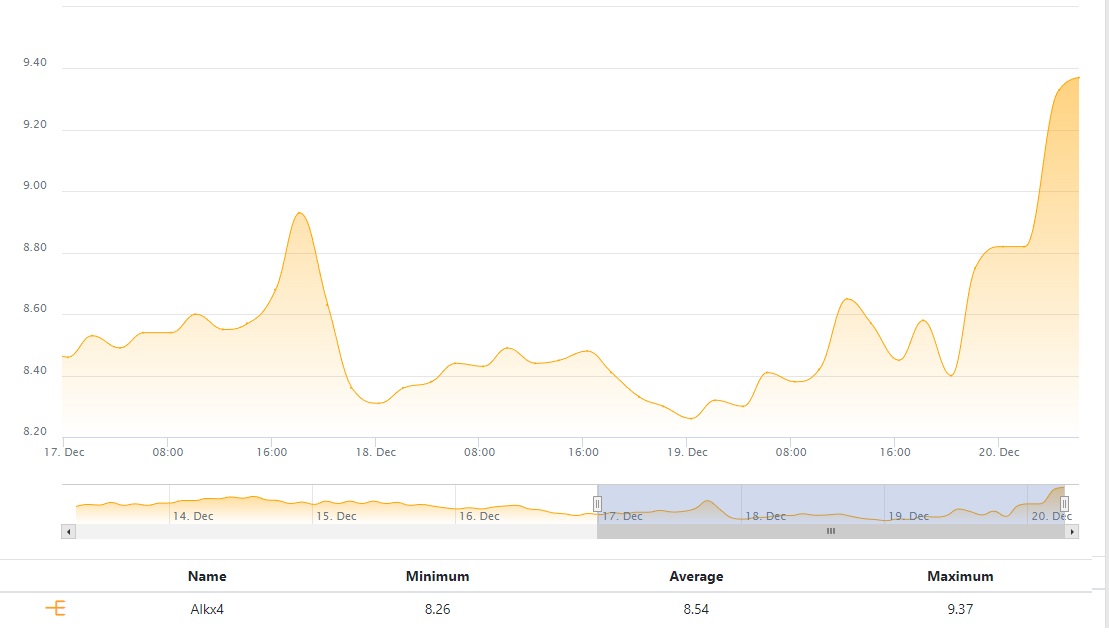
Lastly, pH:
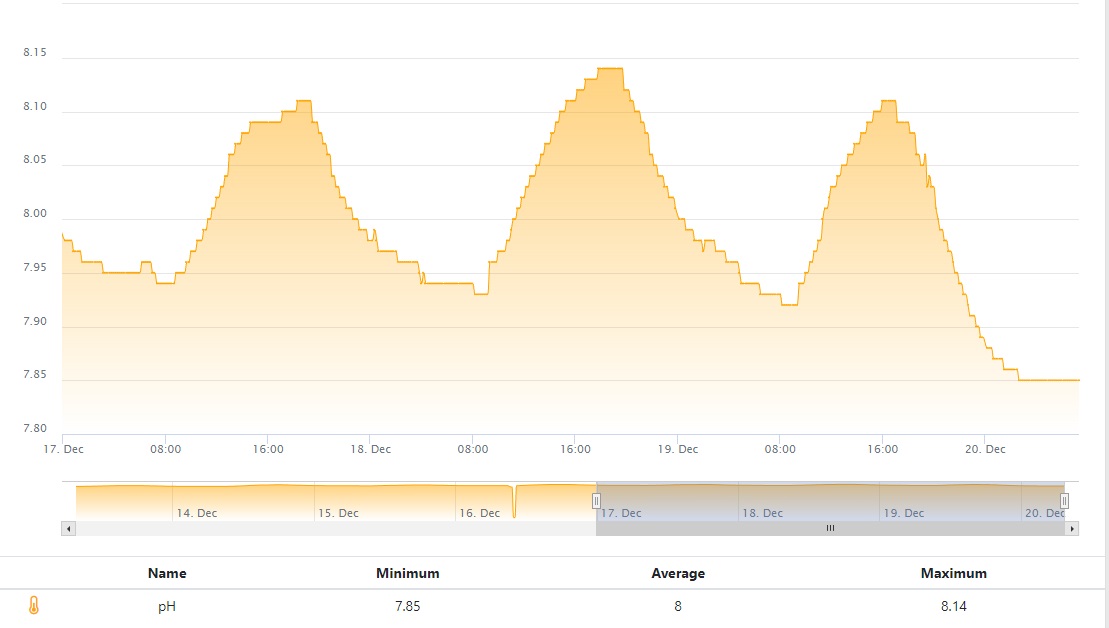
None of this is surprising. Given my understanding (and I could be wrong), it's exactly how I'd expect the tank to react given the increase in dissolved Co2.
Things I've tried:
1. Co2 scrubber
2. Covering tank with plastic wrap (was an experiment)
3. Opening doors when Co2 increases -- effective but not always an option here in Florida.
4. Setting HVAC fan on "low" to disperse Co2.
5. Increasing Trident sampling time to 12 samples/day (every 2 hours). Also set a very wide control range (1.5 dKh) and dosing limit (50%) to accommodate the swings and maintain Trident control.
Options to be explored:
1. Externally venting the range hood. This is going to occur sometime this year as part of a larger remodel
2. Install an ERV. Exploring this option.
3. External skimmer air intake. A possibility once tank is in a more permanent location (see also: aforementioned remodel). Does nothing to reduce Co2 absorption.
4. Kalkwasser. Not sure if this would increase stability or not. I suspect I'd get more of a pH bump than the AF balling method, but that doesn't reduce the Co2 absorption.
5. Fine-tuning powerhead placement to avoid surface agitation.
6. Increasing Trident sampling frequency to every hour. Expensive -- will need to explore ABC reagents to keep cost down.
I know the best path forward would be an ERV but that's not a quick/easy fix. It's certainly not off the table long-term. Is there anything else I'm missing here? Is this even a problem?
Below are some graphs of data I've collected:
First, Co2 (right side of graph cut-off to maintain date/time consistency across all plots):
Next, alk:
Lastly, pH:
None of this is surprising. Given my understanding (and I could be wrong), it's exactly how I'd expect the tank to react given the increase in dissolved Co2.
Things I've tried:
1. Co2 scrubber
2. Covering tank with plastic wrap (was an experiment)
3. Opening doors when Co2 increases -- effective but not always an option here in Florida.
4. Setting HVAC fan on "low" to disperse Co2.
5. Increasing Trident sampling time to 12 samples/day (every 2 hours). Also set a very wide control range (1.5 dKh) and dosing limit (50%) to accommodate the swings and maintain Trident control.
Options to be explored:
1. Externally venting the range hood. This is going to occur sometime this year as part of a larger remodel
2. Install an ERV. Exploring this option.
3. External skimmer air intake. A possibility once tank is in a more permanent location (see also: aforementioned remodel). Does nothing to reduce Co2 absorption.
4. Kalkwasser. Not sure if this would increase stability or not. I suspect I'd get more of a pH bump than the AF balling method, but that doesn't reduce the Co2 absorption.
5. Fine-tuning powerhead placement to avoid surface agitation.
6. Increasing Trident sampling frequency to every hour. Expensive -- will need to explore ABC reagents to keep cost down.
I know the best path forward would be an ERV but that's not a quick/easy fix. It's certainly not off the table long-term. Is there anything else I'm missing here? Is this even a problem?
Last edited:


















