i am looking at trying Brightwell Aquarics Phosphat-E. The bottle says 1 ml will remove 1ppm of phosphate from 4 gallons. I have a 100 gallon tank with .1 phosphate. If I add 1.25ml 1 time that should drop my phosphate to .05. Is my math correct? What’s the best way to add this? And how often should I add it?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Phosphate remover math help
- Thread starter USMA36
- Start date
- Tagged users None
Flippers4pups
Fins up since 1993
View BadgesExcellence Award
Reef Tank 365
Article Contributor
Reef Squad Emeritus
Hospitality Award
i am looking at trying Brightwell Aquarics Phosphat-E. The bottle says 1 ml will remove 1ppm of phosphate from 4 gallons. I have a 100 gallon tank with .1 phosphate. If I add 1.25ml 1 time that should drop my phosphate to .05. Is my math correct? What’s the best way to add this? And how often should I add it?
Unfortunately I have no experience with this product. Maybe the #reefsquad knows?
Fudsey
Jack of all trades, Master of none ;-)
View Badges
Reef Squad
Excellence Award
Reef Tank 365
Photo of the Month
Hospitality Award
NHFS Member
My Tank Thread
It's like lathium isn't it? @jsker uses that, maybe he would know.
How is it that I get a 90% in calculus but THIS is what gets me?i am looking at trying Brightwell Aquarics Phosphat-E. The bottle says 1 ml will remove 1ppm of phosphate from 4 gallons. I have a 100 gallon tank with .1 phosphate. If I add 1.25ml 1 time that should drop my phosphate to .05. Is my math correct? What’s the best way to add this? And how often should I add it?
Okay nevermind it didn't get me I figured it out and yes your math is correct
ppm reduced = z
gallons = y
ml dosed = x
The function has 2 variables which is why it was so tricky, but the function would be
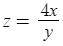
When you plug in 1.25ml as x and 100 gallons in as y you get:
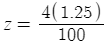
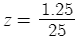
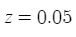
Subtract 0.05 from o.1 (your current phosphates) and you get 0.05ppm as you originally calculated
ppm reduced = z
gallons = y
ml dosed = x
The function has 2 variables which is why it was so tricky, but the function would be
When you plug in 1.25ml as x and 100 gallons in as y you get:
Subtract 0.05 from o.1 (your current phosphates) and you get 0.05ppm as you originally calculated
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,953
FWIW, phosphate calculations are essentially useless. If you are at 1 ppm in a reef tank and replace 100% of the water with zero phosphate water, you may end up with a high phosphate value again because a large amount of phospahte can come off the rock and sand, and the opposite effect works when dosing it.
Are you saying the math used above to determine dosage of Lanthium Chloride (Brightwell Aquatics Phosphat-E) will not be accurate?FWIW, phosphate calculations are essentially useless. If you are at 1 ppm in a reef tank and replace 100% of the water with zero phosphate water, you may end up with a high phosphate value again because a large amount of phospahte can come off the rock and sand, and the opposite effect works when dosing it.
What makes me wonder then is why they include the information 1ml in 4 gallons reduces 1ppm if it's pointless to calculate anywaysAre you saying the math used above to determine dosage of Lanthium Chloride (Brightwell Aquatics Phosphat-E) will not be accurate?
I think what Randy meant is that it’ll take out 1 ppm but it’ll Leach from the rock back into the water column until it’s balanced out again so you’ll end up with a higher number than you thought you were going to get.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,953
Are you saying the math used above to determine dosage of Lanthium Chloride (Brightwell Aquatics Phosphat-E) will not be accurate?
It is almost certainly inaccurate regardless (even if done correctly, it assumes no other sink for lanthanum than lanthanum phosphate), but it is intended (if that is the correct word for misuse of chemistry) for lowering phosphate in water only, not where most of the phosphate is bound to rock and sand that acts as a big buffer.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,953
I think what Randy meant is that it’ll take out 1 ppm but it’ll Leach from the rock back into the water column until it’s balanced out again so you’ll end up with a higher number than you thought you were going to get.
yes, that's the most obvious issue.
Even though your phosphate drop will almost certainly be less than calculated, you should still be pretty careful with dosing lanthanum directly into your DT. You are looking at attempting to generate a pretty small desired drop in phosphate level with a relatively aggressive technique. Use less than recommended, measure the result the next day, and use again if necessary. I'd also try to avoid allowing the precipitate to circulate as much as possible, as some fish are reportedly sensitive to it. Dripping it drop by drop directly into a 1 micron mesh filter sock in the sump seems to work for some.
Another way to think of the math: 25 ml will drop the phosphate in 100 gallons by 1 ppm. You want a drop of 1/20th of that. 25/20 = 1.25 ml.
Another way to think of the math: 25 ml will drop the phosphate in 100 gallons by 1 ppm. You want a drop of 1/20th of that. 25/20 = 1.25 ml.
Similar threads
- Replies
- 11
- Views
- 351
- Replies
- 38
- Views
- 844
- Replies
- 27
- Views
- 494
New Posts
-
-
-
Sexual dimorphism identification of Chelmon rostratus, the Copperband Butterfly,
- Latest: MalteserReefer
-
-
















