- Joined
- May 22, 2016
- Messages
- 6,566
- Reaction score
- 10,146
So, given some time and given enough charted data, we may all be in possession of universal Hanna checkers?
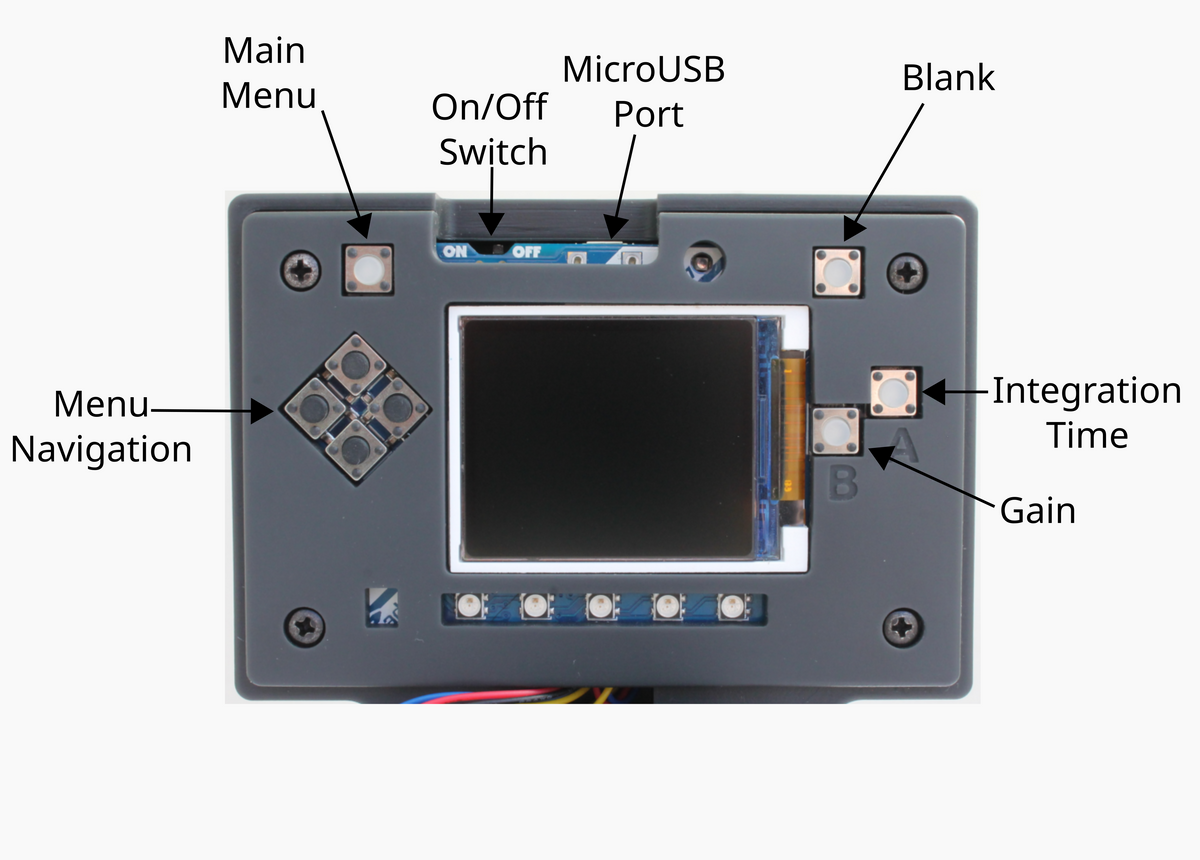
If you wanted a universal Hanna checker, you'd get a colorimeter (or spectrometer) instead of a half dozen checkers.So, you're saying there's a chance...