Hey! Not a chemist so I need a bit of help with a tiny issue, I have used Sodium Thiosulfate in the past for neutralising bleach and it was in large crystal form almost like carbon pellet shape, however I needed some now and ordered pentahydrate by mistake which is pretty much a powder form, my issue is that the recipe I’ve used and know is for the crystal form and I’m not sure if the mixing quantity is different for the pentahydrate, I’m kinda considering to just re-order but i have a kilo of the stuff now. Maybe someone can help? @Randy Holmes-Farley
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Sodium Thiosulfate pentahydrate
- Thread starter Mad Doc
- Start date
- Tagged users None
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
Sodium thiosulfate pentahydrate (molecular weight = 248 g/mole) just has 5 extra water molecules in the crystal, relative to anhydrous sodium thiosulfate (molecular weight = 158 g/mole).
To use the former to get the same strength as the latter, you need to use 248/158 = 1.6 times as much.
To use the former to get the same strength as the latter, you need to use 248/158 = 1.6 times as much.
Thank you very much Randy!!! I’m assuming it should have no difference in the way it dissolves?Sodium thiosulfate pentahydrate (molecular weight = 248 g/mole) just has 5 extra water molecules in the crystal, relative to anhydrous sodium thiosulfate (molecular weight = 158 g/mole).
To use the former to get the same strength as the latter, you need to use 248/158 = 1.6 times as much.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
Thank you very much Randy!!! I’m assuming it should have no difference in the way it dissolves?
Dissolution might take a different amount of time, but the ions in solution after dissolution are identical.
Thank you couldn’t find any information on the above and it took you like 5 sec to figure it out so I’m very greatful!Dissolution might take a different amount of time, but the ions in solution after dissolution are identical.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
Thank you couldn’t find any information on the above and it took you like 5 sec to figure it out so I’m very greatful!
lol
You’re welcome. Happy Reefing!
Don’t wanna
 can that be used or try to find something better UK is pretty lame for certain things.
can that be used or try to find something better UK is pretty lame for certain things.
become a pain but just came across another issue which I thought would be the easiest part. I can’t seem to find household bleach 5% sodium hypochlorite the only ones I’m finding that are unscented describe it as containing 1g to 100g sodium hypochlorite whatever that means but doesn’t make sense to me. Definitely doesn’t seem like 5%.lol
You’re welcome. Happy Reefing!
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
Don’t wanna
become a pain but just came across another issue which I thought would be the easiest part. I can’t seem to find household bleach 5% sodium hypochlorite the only ones I’m finding that are unscented describe it as containing 1g to 100g sodium hypochlorite whatever that means but doesn’t make sense to me. Definitely doesn’t seem like 5%.can that be used or try to find something better UK is pretty lame for certain things.
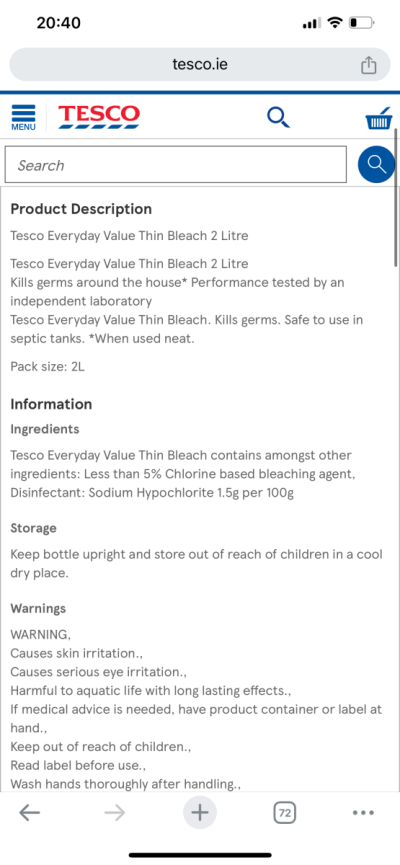
Do you have the exact ingredient description? Thats a ratio of ingredients, not the concentration. That’s probably sodium hydroxide and sodium hypochlorite .
That’s basically all there is on the bottleDo you have the exact ingredient description? Thats a ratio of ingredients, not the concentration. That’s probably sodium hydroxide and sodium hypochlorite .
Attachments
Also this was my other option but they both make no sense lolThat’s basically all there is on the bottle
Attachments
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
I guess that first one is a concentration, but it seems very low at 1%. Use 5x as much of it as 5% bleach and you should be OK if you are using it in an application where it gets combined with additional water.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
The second one is 1.5%.
okay so if I’m correct this means I should use the Sodium thiosulfate as if I was using 5 times less bleach since I’m only doing it x5 coz of its strength?
If I need 20ml of bleach normally and then need 5ml of Sodium Thiosulfate to negate this, should I still use 5ml even tho I’m using 100ml of the 1% bleach or dose 25ml Sodium Thiosulfate instead?
P.S. I really do appreciate your help sir, thank you!
If I need 20ml of bleach normally and then need 5ml of Sodium Thiosulfate to negate this, should I still use 5ml even tho I’m using 100ml of the 1% bleach or dose 25ml Sodium Thiosulfate instead?
P.S. I really do appreciate your help sir, thank you!
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,783
Yes, that’s what I would do.okay so if I’m correct this means I should use the Sodium thiosulfate as if I was using 5 times less bleach since I’m only doing it x5 coz of its strength?
If I need 20ml of bleach normally and then need 5ml of Sodium Thiosulfate to negate this, should I still use 5ml even tho I’m using 100ml of the 1% bleach or dose 25ml Sodium Thiosulfate instead?
P.S. I really do appreciate your help sir, thank you!
The second one is 1.5
Similar threads
- Replies
- 19
- Views
- 544
- Replies
- 9
- Views
- 666
- Replies
- 25
- Views
- 643