Calcium reactors are perhaps the best method for providing calcium and alkalinity in reef aquariums with a medium to high calcification demand. It is a system that does not introduce unnecessary ions, does not depend on evaporation rates and has very low operating costs, with a significant safety. The reactor consists of one or more chambers, which are filled with a medium consisting of calcium carbonate and optionally magnesium carbonate. Aquarium water previously mixed with CO2 is circulated through the reactor. The carbonic acid which is formed dissolves the medium, returning calcium, bicarbonate and magnesium ions to the water in high concentrations.
These high concentrations are achieved because the injected CO2 lowers the pH inside the chamber, typically to 6.5-6.2, which prevents precipitation. A small water flow from the aquarium, which circulates through the reactor and returns back, provides the necessary amounts of calcium, magnesium and alkalinity. The pH at the outlet of the reactor is typically between 6.2 and 6.5, which can sometimes cause a slight drop in the aquarium pH. Some aquarists combine a calcium reactor with a kalkwasser reactor for this reason.
Regardless of the technology, the principle of operation is always the same. The system make the coral calcification process reversible by lowering the pH with carbonic acid.The reaction is described as follows .Carbon dioxide injected into the water forms carbonic acid, which dissolves the calcium carbonate, releasing calcium and bicarbonate ions:
CaCO3 (calcium carbonate) + H2O (water) + CO2 (carbon dioxide) ⇨
⇨ Ca2+ (calcium ions) + 2⋅HCO3- (bicarbonate ions)
These bicarbonate ions subsequently enter the aquarium, joining the carbonate/bicarbonate system that determines the alkalinity .A calcium reactor provides a continuous flow of calcium and alkalinity, replenishing what is consumed. Traditional equipment provides effluent alkalinity values around 45 ºdKH, while more advanced equipment delivers values between 60 ºdKH and 90 ºdKH. To satisfy the aquarium calcification demand, the reactor is configured with certain flow rate and effluent alkalinity value. To increase the reactor´s effluent alkalinity, the amount of injected CO2 must be increased, or the flow rate decreased within certain limits.
Regardless of their design, all reactors require a pressurized bottle containing carbon dioxide. This can be purchased from the local fish store, or medical supply companies. It is important to use good quality gas, to avoid introducing contaminants into the water. The CO2 stored in the bottle must reduce its pressure before entering the reactor. For this purpose, a pressure reducer with a pressure indicator is used. The efficiency of calcium reactors has increased over time, from the traditional models, controlled by a pH probe, to the most modern ones, which use optical probes, level probes and electronic controllers. Let us look at some examples.
Figures 1 and 2 show the assembly of a pH probe controlled reactor and its main components. We can highlight the following: solenoid valve, bubble counter, pH probe, first chamber, second chamber, internal recirculation pump, flow pump, CO2 chamber, and flow meter. The solenoid valve is connected to the gas outlet of the pressure reducer and is operated by a pH controller, whose probe is inserted in the upper part of the first chamber. The probe sends the pH value in real time to the controller, which opens the solenoid valve when it rises above a set point, e.g. 6.3. When carbonic acid concentration inside the chamber decreases, pH increases and the solenoid valve opens, injecting more CO2.
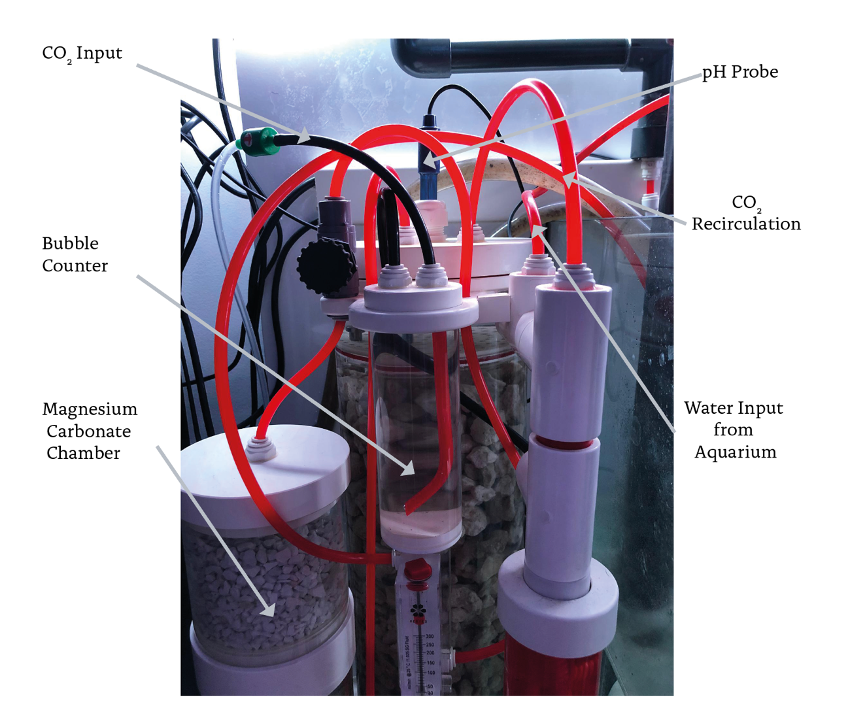
Figure 1. Calcium reactor controlled by pH probe.
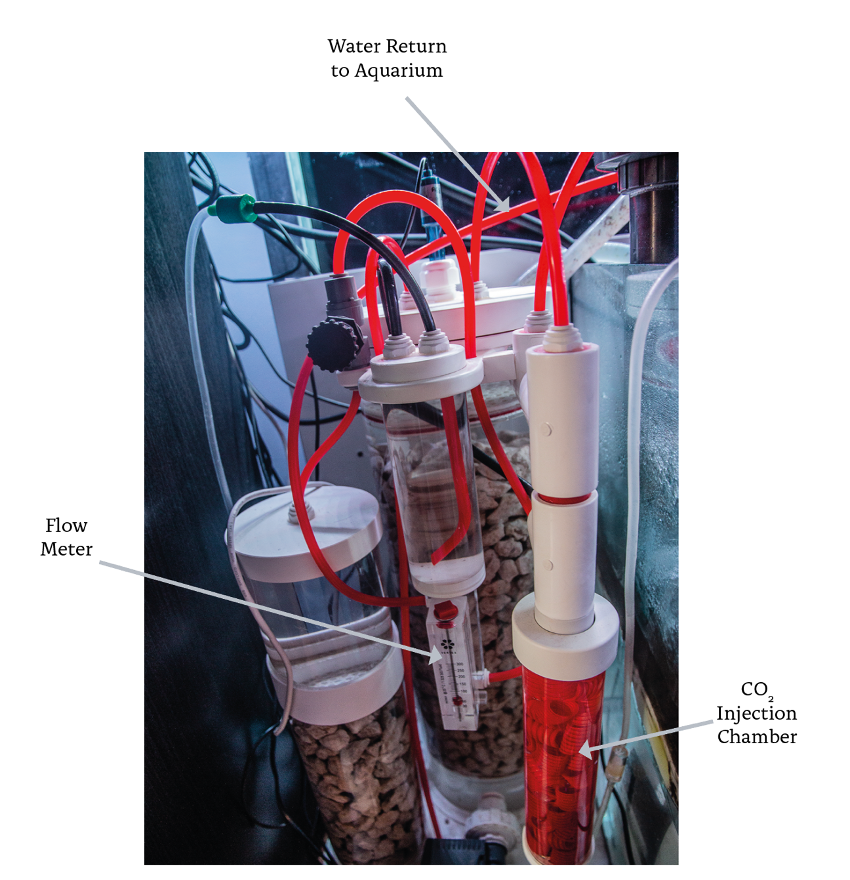
Figure 2. Calcium Reactor controlled by pH probe.

Figure 3. Pressure reducer.
With the pressure reducer (figure 3), the inlet CO2 flow is manually regulated and does not change until the solenoid valve is activated. This flow is quantified by the number of bubbles per minute that circulate through the meter. The second chamber is used to hold magnesium carbonate or an additional volume of calcium carbonate. This chamber also serves as a “degassing” stage, because the water is discharged of carbonic acid as it passes through it. Figure 4 shows the two water flows inside the equipment. R flow shows the path that the water follows to dissolve the carbonate medium, while C flow shows the path traveled by the aquarium water through the reactor.
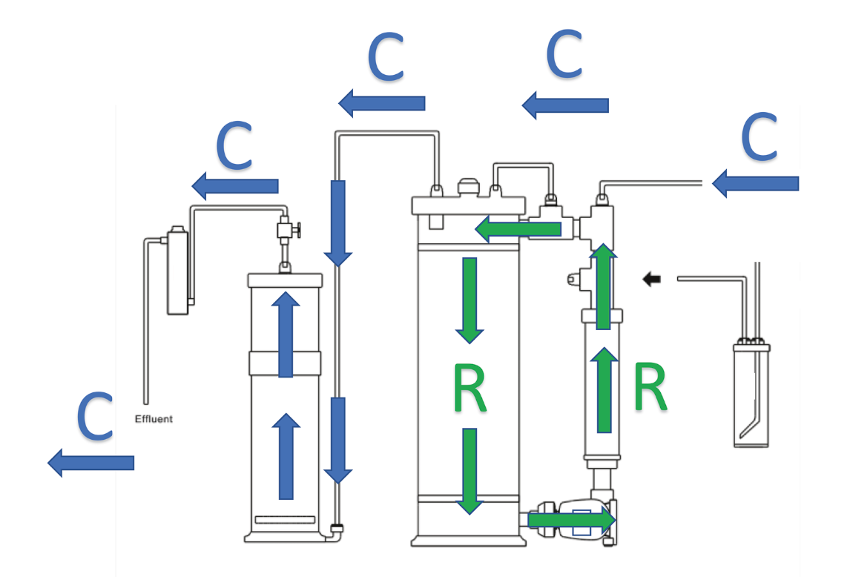
Figure 4. Water flows inside a calcium reactor.
Figure 4 shows the two water flows inside the equipment. R flow shows the path that the water follows to dissolve the carbonate medium, while C flow shows the path traveled by the aquarium water through the reactor.C flow determines, to a large extent, the daily rate of calcium and alkalinity input. As indicated above, the water from the aquarium displaces the carbonic acid water contained in the unit, increasing the pH. This increase is detected by the probe and the controller activates the solenoid valve to inject more CO2.The total daily amount of alkalinity supplied by the reactor to the aquarium depends on two factors: its flow rate and the effluent alkalinity value. It is possible to adjust only one of them or a combination of both. As we have indicated, the effluent alkalinity value is increased by injecting a higher CO2 flow or by decreasing the water flow rate to allow a higher saturation of the gas inside the reactor.
Any calcium reactor requires a calibration period prior to start-up. The calibration serves to adjust the capacity of the equipment to maintain the aquarium calcium and alkalinity levels of without decreasing its pH. It is important to note that the adjustments made to the equipment impact both calcium and alkalinity, and it is not possible to modify them independently. To make independent corrections, it is necessary to use chemical additives.Before calibrating the reactor, the daily alkalinity consumption of the aquarium, i.e. the alkalinity demand, should be measured. Once this is available, the administration of any additives is stopped and the reactor is switched on at a standard setting, depending on the model, e.g. a flow rate of 1 liter per hour and 15 bubbles per minute. After 24 hours, the alkalinity of the aquarium is measured and the alkalinity value of the previous day is subtracted. If the result is negative, the reactor needs to be adjusted to provide a higher alkalinity rate. The complete calibration of the reactor may take days or even weeks, depending on the model and the calcification demand of the aquarium.
In figures 5 and 6, a calcium reactor controlled by optical probe is depicted. The optical probe measures the amount of injected CO2, sending a signal to the electronic controller when a gas deficiency is detected. The controller opens the solenoid valve and more CO2 enters the system. A peristaltic pump connected to the controller, regulate the water flow through the reactor (C flow). In addition to the pressurized parts, it consists of the following elements: CO2 chamber, first chamber, second chamber, optical probe, electronic controller with touch screen and recirculation pump.

Figure 5. Calcium reactor controlled by optical probe.

Figure 6. Calcium reactor controlled by optical probe.
Reactors controlled by optical probe are very efficient in measuring the amount of injected CO2 .The sensor detects the absence and presence of bubbles, sending a signal to the controller that activates the solenoid valve. This extraordinary efficiency makes the dissolved CO2 content very high, producing an effluent pH around 6.2. Effluent alkalinity values are typically between 60 ºdKH and 90 ºdKH. The controller has several means of operation that allow for greater efficiency and convenience, for example, the degassing mode, which circulates the internal water to remove unused CO2 that has been trapped inside the unit. The flow rate in ml/hour is selected on the touch screen, thus regulating the daily rate of alkalinity supplied to the aquarium.
These high concentrations are achieved because the injected CO2 lowers the pH inside the chamber, typically to 6.5-6.2, which prevents precipitation. A small water flow from the aquarium, which circulates through the reactor and returns back, provides the necessary amounts of calcium, magnesium and alkalinity. The pH at the outlet of the reactor is typically between 6.2 and 6.5, which can sometimes cause a slight drop in the aquarium pH. Some aquarists combine a calcium reactor with a kalkwasser reactor for this reason.
Regardless of the technology, the principle of operation is always the same. The system make the coral calcification process reversible by lowering the pH with carbonic acid.The reaction is described as follows .Carbon dioxide injected into the water forms carbonic acid, which dissolves the calcium carbonate, releasing calcium and bicarbonate ions:
CaCO3 (calcium carbonate) + H2O (water) + CO2 (carbon dioxide) ⇨
⇨ Ca2+ (calcium ions) + 2⋅HCO3- (bicarbonate ions)
These bicarbonate ions subsequently enter the aquarium, joining the carbonate/bicarbonate system that determines the alkalinity .A calcium reactor provides a continuous flow of calcium and alkalinity, replenishing what is consumed. Traditional equipment provides effluent alkalinity values around 45 ºdKH, while more advanced equipment delivers values between 60 ºdKH and 90 ºdKH. To satisfy the aquarium calcification demand, the reactor is configured with certain flow rate and effluent alkalinity value. To increase the reactor´s effluent alkalinity, the amount of injected CO2 must be increased, or the flow rate decreased within certain limits.
Regardless of their design, all reactors require a pressurized bottle containing carbon dioxide. This can be purchased from the local fish store, or medical supply companies. It is important to use good quality gas, to avoid introducing contaminants into the water. The CO2 stored in the bottle must reduce its pressure before entering the reactor. For this purpose, a pressure reducer with a pressure indicator is used. The efficiency of calcium reactors has increased over time, from the traditional models, controlled by a pH probe, to the most modern ones, which use optical probes, level probes and electronic controllers. Let us look at some examples.
Figures 1 and 2 show the assembly of a pH probe controlled reactor and its main components. We can highlight the following: solenoid valve, bubble counter, pH probe, first chamber, second chamber, internal recirculation pump, flow pump, CO2 chamber, and flow meter. The solenoid valve is connected to the gas outlet of the pressure reducer and is operated by a pH controller, whose probe is inserted in the upper part of the first chamber. The probe sends the pH value in real time to the controller, which opens the solenoid valve when it rises above a set point, e.g. 6.3. When carbonic acid concentration inside the chamber decreases, pH increases and the solenoid valve opens, injecting more CO2.
Figure 1. Calcium reactor controlled by pH probe.
Figure 2. Calcium Reactor controlled by pH probe.
Figure 3. Pressure reducer.
With the pressure reducer (figure 3), the inlet CO2 flow is manually regulated and does not change until the solenoid valve is activated. This flow is quantified by the number of bubbles per minute that circulate through the meter. The second chamber is used to hold magnesium carbonate or an additional volume of calcium carbonate. This chamber also serves as a “degassing” stage, because the water is discharged of carbonic acid as it passes through it. Figure 4 shows the two water flows inside the equipment. R flow shows the path that the water follows to dissolve the carbonate medium, while C flow shows the path traveled by the aquarium water through the reactor.
Figure 4. Water flows inside a calcium reactor.
Figure 4 shows the two water flows inside the equipment. R flow shows the path that the water follows to dissolve the carbonate medium, while C flow shows the path traveled by the aquarium water through the reactor.C flow determines, to a large extent, the daily rate of calcium and alkalinity input. As indicated above, the water from the aquarium displaces the carbonic acid water contained in the unit, increasing the pH. This increase is detected by the probe and the controller activates the solenoid valve to inject more CO2.The total daily amount of alkalinity supplied by the reactor to the aquarium depends on two factors: its flow rate and the effluent alkalinity value. It is possible to adjust only one of them or a combination of both. As we have indicated, the effluent alkalinity value is increased by injecting a higher CO2 flow or by decreasing the water flow rate to allow a higher saturation of the gas inside the reactor.
Any calcium reactor requires a calibration period prior to start-up. The calibration serves to adjust the capacity of the equipment to maintain the aquarium calcium and alkalinity levels of without decreasing its pH. It is important to note that the adjustments made to the equipment impact both calcium and alkalinity, and it is not possible to modify them independently. To make independent corrections, it is necessary to use chemical additives.Before calibrating the reactor, the daily alkalinity consumption of the aquarium, i.e. the alkalinity demand, should be measured. Once this is available, the administration of any additives is stopped and the reactor is switched on at a standard setting, depending on the model, e.g. a flow rate of 1 liter per hour and 15 bubbles per minute. After 24 hours, the alkalinity of the aquarium is measured and the alkalinity value of the previous day is subtracted. If the result is negative, the reactor needs to be adjusted to provide a higher alkalinity rate. The complete calibration of the reactor may take days or even weeks, depending on the model and the calcification demand of the aquarium.
In figures 5 and 6, a calcium reactor controlled by optical probe is depicted. The optical probe measures the amount of injected CO2, sending a signal to the electronic controller when a gas deficiency is detected. The controller opens the solenoid valve and more CO2 enters the system. A peristaltic pump connected to the controller, regulate the water flow through the reactor (C flow). In addition to the pressurized parts, it consists of the following elements: CO2 chamber, first chamber, second chamber, optical probe, electronic controller with touch screen and recirculation pump.
Figure 5. Calcium reactor controlled by optical probe.
Figure 6. Calcium reactor controlled by optical probe.
Reactors controlled by optical probe are very efficient in measuring the amount of injected CO2 .The sensor detects the absence and presence of bubbles, sending a signal to the controller that activates the solenoid valve. This extraordinary efficiency makes the dissolved CO2 content very high, producing an effluent pH around 6.2. Effluent alkalinity values are typically between 60 ºdKH and 90 ºdKH. The controller has several means of operation that allow for greater efficiency and convenience, for example, the degassing mode, which circulates the internal water to remove unused CO2 that has been trapped inside the unit. The flow rate in ml/hour is selected on the touch screen, thus regulating the daily rate of alkalinity supplied to the aquarium.
Last edited by a moderator:












