- Joined
- Sep 13, 2018
- Messages
- 360
- Reaction score
- 333
Hey everybody, My tank's demand has been steadily spiraling out of control. It's gotten to the point where my DOS pumps are too noisy and I'm getting tired of mixing up two part. I feel I've covered all my bases but I'm looking for some additional suggestions for troubleshooting. Is there any way my corals are actually using 5.2dkh per day? Or is it just the "cost of doing business" to keep a proper pH? I've gone from 200ml a day to 550ml over the past 4 months.
I've had reef tanks since 04/05 but just recently REALLY started getting back into the hobby. the first two years getting back at it were ROUGH plagued by horrific algae, dinoflagellates and sudden mysterious "mini crashes" where acros STN'd and lost a clam. My current 150 gallon reef was set up 14 months ago and the dino continued to plague me despite having good luck with coral health. Maintaining a proper pH has always been an issue and I was suspicious that high CO2 levels were the root cause of all my troubles. October 1st I started a series of experiments inspired by Randy's article here https://www.reef2reef.com/threads/alkalinity-stability-ph-stability-are-they-even-different.711768/ . My end goal was to maintain a stable alkalinity as well as a stable, proper pH. It's been an interesting journey and I learned a LOT in the process. I'm happy to say the results are better than I could've ever imagined. previously I was lucky to average an 8.00pH with up to a .5 daily swing and pH as low as 7.58. Now my pH is 8.3 +/- 0.06 and alkalinity 9.1DKH +/- 0.3. Corals and my clam are absolutely THRIVING with some growing over an inch per month. Dino is gone and the last time I was this happy with my reef was 2007.
My alkatronic tests very frequently and communicates to the apex. I toggle between Randy's standard soda ash solution and Lye solution based on the apex's pH. Above 8.25pH it doses soda ash. Below it doses Lye. Several throttle outlets fine tune the dosage. When I'm away for the weekend the system will automatically transition to dosing 95% soda ash with just 20-40ml lye. I recently added another dos for bicarbonate. Right now I'm just using it to dampen peak pH and buffer up alk with 45ml between 11p and midnight. I also added a CO2 meter. in the future I may develop a way to integrate everything. for now, it's nice to have a backup alkalinity solution on tap... I allowed my calcium and soda ash jug to go empty a few weeks ago while away. I was able to limp through with a little bit of lye dosing and cutting lights but in the end, I missed about 12 hours worth of dosing and alk dropped over 2DKH. I see that as a sign the demand is real however I may be mistaken. It's important to note that my Alkatronic tests a consistent .6-.7dkh higher than it actually is. Confirmed with many salifert kits, hanna and Randy's alkalinity reference solution. all of my alkalinity solutions dose in the sump, directly in front of a maxijet 1200. aside from a little bit of splashing, I NEVER see any signs of precipitation. Heaters and pumps are always squeaky clean and sand isn't clumping anywhere.
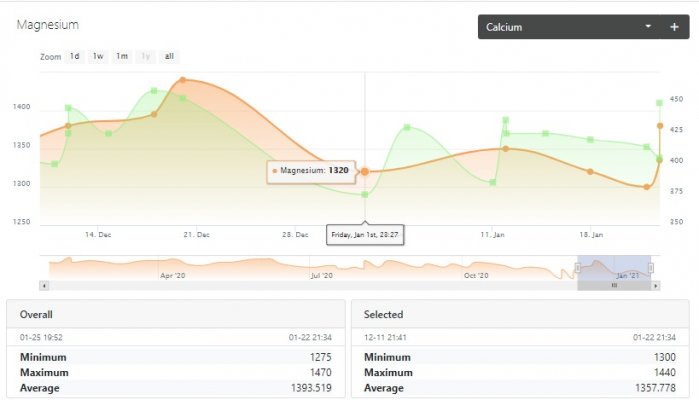
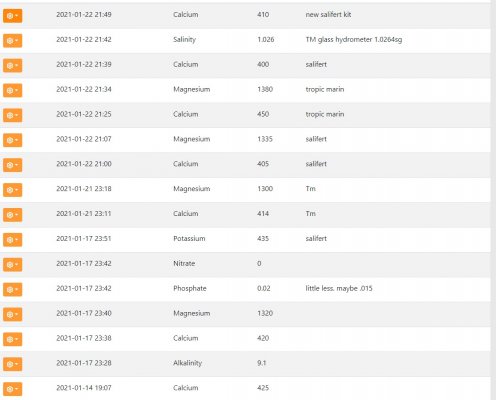
My calcium solution is double strength(1000g/ gallon) and I periodically adjust it to match half of the total soda ash/lye dosing. it doses directly to the display. I dosed 34-45ppm of magnesium last night followed by an additional manual dose of 10ppm calcium. This is the first time I've ever dosed magnesium. Normally water changes handle the demand but I've had to cut those out recently as nutrients have tanked. Today, test results mostly agreed with each other and rose as expected. It all seems to be within claimed tolerances with the exception of the 450ppm TM calcium reading... I don't like making changes too fast but I feel I need to do something... either lower my alkalinity or raise my magnesium even higher. This level of demand seems impossible but trial and error leads me to believe it's correct. Any ideas???


I've had reef tanks since 04/05 but just recently REALLY started getting back into the hobby. the first two years getting back at it were ROUGH plagued by horrific algae, dinoflagellates and sudden mysterious "mini crashes" where acros STN'd and lost a clam. My current 150 gallon reef was set up 14 months ago and the dino continued to plague me despite having good luck with coral health. Maintaining a proper pH has always been an issue and I was suspicious that high CO2 levels were the root cause of all my troubles. October 1st I started a series of experiments inspired by Randy's article here https://www.reef2reef.com/threads/alkalinity-stability-ph-stability-are-they-even-different.711768/ . My end goal was to maintain a stable alkalinity as well as a stable, proper pH. It's been an interesting journey and I learned a LOT in the process. I'm happy to say the results are better than I could've ever imagined. previously I was lucky to average an 8.00pH with up to a .5 daily swing and pH as low as 7.58. Now my pH is 8.3 +/- 0.06 and alkalinity 9.1DKH +/- 0.3. Corals and my clam are absolutely THRIVING with some growing over an inch per month. Dino is gone and the last time I was this happy with my reef was 2007.
My alkatronic tests very frequently and communicates to the apex. I toggle between Randy's standard soda ash solution and Lye solution based on the apex's pH. Above 8.25pH it doses soda ash. Below it doses Lye. Several throttle outlets fine tune the dosage. When I'm away for the weekend the system will automatically transition to dosing 95% soda ash with just 20-40ml lye. I recently added another dos for bicarbonate. Right now I'm just using it to dampen peak pH and buffer up alk with 45ml between 11p and midnight. I also added a CO2 meter. in the future I may develop a way to integrate everything. for now, it's nice to have a backup alkalinity solution on tap... I allowed my calcium and soda ash jug to go empty a few weeks ago while away. I was able to limp through with a little bit of lye dosing and cutting lights but in the end, I missed about 12 hours worth of dosing and alk dropped over 2DKH. I see that as a sign the demand is real however I may be mistaken. It's important to note that my Alkatronic tests a consistent .6-.7dkh higher than it actually is. Confirmed with many salifert kits, hanna and Randy's alkalinity reference solution. all of my alkalinity solutions dose in the sump, directly in front of a maxijet 1200. aside from a little bit of splashing, I NEVER see any signs of precipitation. Heaters and pumps are always squeaky clean and sand isn't clumping anywhere.
My calcium solution is double strength(1000g/ gallon) and I periodically adjust it to match half of the total soda ash/lye dosing. it doses directly to the display. I dosed 34-45ppm of magnesium last night followed by an additional manual dose of 10ppm calcium. This is the first time I've ever dosed magnesium. Normally water changes handle the demand but I've had to cut those out recently as nutrients have tanked. Today, test results mostly agreed with each other and rose as expected. It all seems to be within claimed tolerances with the exception of the 450ppm TM calcium reading... I don't like making changes too fast but I feel I need to do something... either lower my alkalinity or raise my magnesium even higher. This level of demand seems impossible but trial and error leads me to believe it's correct. Any ideas???