I suggested a number of changes to that article and none were made. It's been years since I've looked at that graph but no a Stokes Shift doesn't work backwards. I'll review it and try to recall what Dan did and report back.What's up with the handful of points on the wrong side of the excitation - emission diagonal line?
There's a few points plotted there that have shorter wavelength emission peaks then the light they absorb. Is that accurate? Are they like catching two photons and emitting one?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
A Spectrophotometer view of Photosynthetic Organisms in our Tanks
- Thread starter taricha
- Start date
- Tagged users None
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
Apparently concerning the real Green Fluorescent Protein from a. victoria "...The fluorescence quantum yield (QY) of GFP is 0.79." which means for every 100 absorbed (blue) photons, 79 (green) photons are emitted. That's higher than I expected.Interesting thought about the light emission of one protein being absorb by another and causing a secondary emission. Is there something like an efficiency factor that would be make or break this idea? I assume not every photon would find its way to the right place. Can it actually happen? Would we actually be able to observe it?
and apparently the jellyfish emits bioluminescence through the pigment aequorin and "...GFP is co-expressed with aequorin in small granules around the rim of the jellyfish bell. The secondary excitation peak (480 nm) of GFP does absorb some of the blue emission of aequorin, giving the bioluminescence a more green hue."
wikipedia source
Something similar happens a lot in photosynthetic pigments: Fucoxanthin in diatoms and Peridinin in dinos hand off their energy to Chlorophyll, but the connection is tighter than playing catch with a photon from one molecule to another.
FRET = forster (fluorescence) resonant energy transfer is the term for this. Jorg Wiedenmann has done a bunch of cool stuff with coral fluorescent pigments. This paper is about (blue-absorbing) Green fluorescent pigments in corals using increasing amounts of FRET to become emitters of red light in a deep zone where the only available light is blue.
A couple comments: The protein from Aequoria victoria is only remotely related to those proteins found in anthozoans. It has a high quantum yield and requires calcium as a cofactor. Although metal concentrations can affect protein emission, they are not required for chromophore or fluorophore formation. Most quantum yields are much lower - ~0.26 to ~0.5. The relay of energy from one protein to another (where emission is also excitation) can occur but is thought to be rare. Admittedly, I haven't looked at recent papers - my attention has been elsewhere.
I looked at my database. I didn't see any typos there that would suggest emission wavelength is shorter than excitation. So, not sure what's going on with that chart.
I looked at my database. I didn't see any typos there that would suggest emission wavelength is shorter than excitation. So, not sure what's going on with that chart.
Forgot - this article discusses quantum yield, FRET, in minor detail.
https://www.advancedaquarist.com/2006/9/aafeature
https://www.advancedaquarist.com/2006/9/aafeature
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
I suggested a number of changes to that article and none were made. It's been years since I've looked at that graph but no a Stokes Shift doesn't work backwards.
Your annoyance here makes total sense.
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
So I've gotten fortunate and had a few organism I had wanted to sample show up in my systems. Had some Gracilaria pop up in a small test tank.

So I thought I'd revisit the question of red algae.
Here's my absorption spectrum for the gracilaria
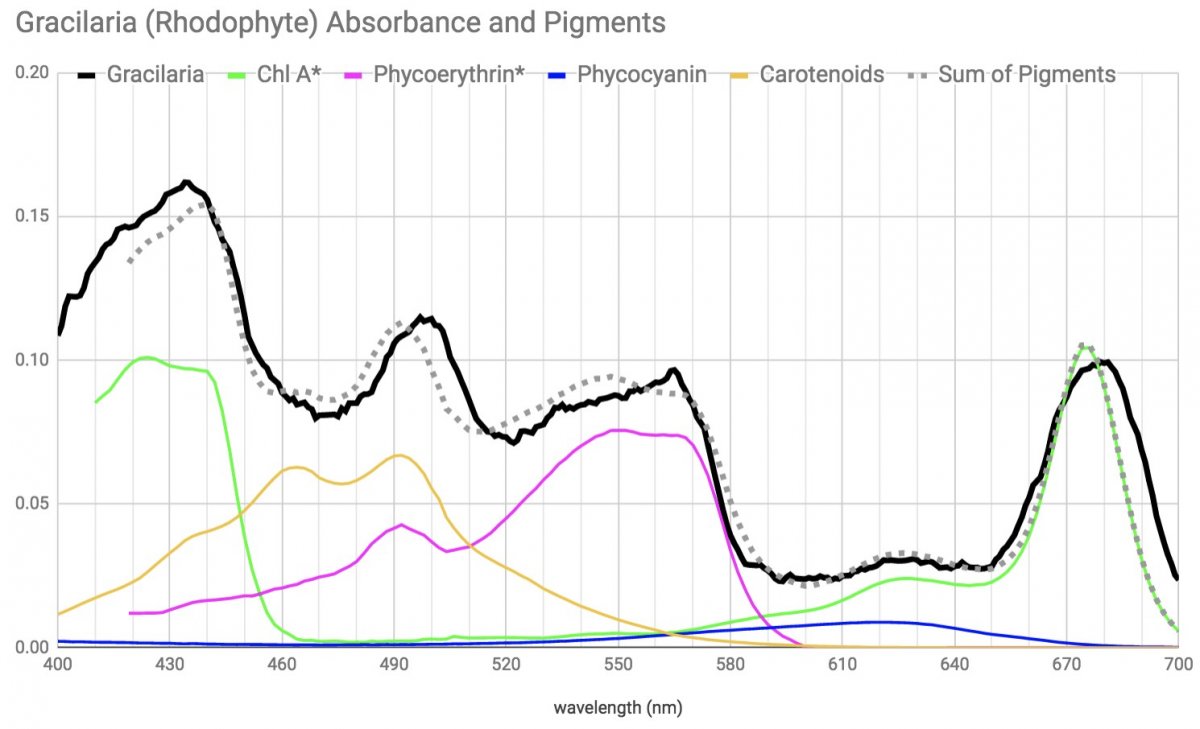
Note the phycoerythrin and even a little phycocyanin (confirmed by fluorescence).
This paper provides absorption spectra and action spectra for several red algae and this from porphyrella has an absorption that is a very close match.
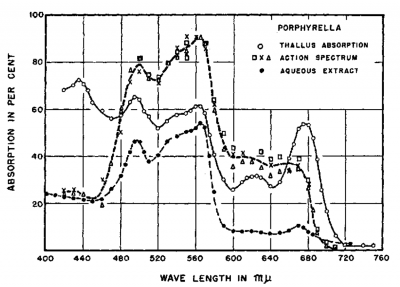
But as in the action spectrum Dana posted earlier, Chlorophyll absorbance correlates to low action and is much less of a driver of the action than the phycoerythrin (and phycocyanin).
to quote the paper:
"In green and brown algae, light absorbed by both chlorophyll and carotenoids seems photosynthetically effective, although some inactive absorption by carotenoids is indicated. Action spectra for a wide variety of red algae, however, show marked deviations from their corresponding absorption spectra. The photosynthetic rates are high in the spectral regions absorbed by the water-soluble "phycobilin" pigments (phycoerythrin and phycocyanin), while the light absorbed by chlorophyll and carotenoids is poorly utilized for oxygen production."
Edit: so if the action spectra are to be trusted we need to provide light in the green and understand that it's not benefitting from our normal Blue and Red light scheme.

So I thought I'd revisit the question of red algae.
... or do I need to consider the correct spectrum for Gracilaria?
I'll toss these into the mix:
Here's my absorption spectrum for the gracilaria
Note the phycoerythrin and even a little phycocyanin (confirmed by fluorescence).
This paper provides absorption spectra and action spectra for several red algae and this from porphyrella has an absorption that is a very close match.

But as in the action spectrum Dana posted earlier, Chlorophyll absorbance correlates to low action and is much less of a driver of the action than the phycoerythrin (and phycocyanin).
to quote the paper:
"In green and brown algae, light absorbed by both chlorophyll and carotenoids seems photosynthetically effective, although some inactive absorption by carotenoids is indicated. Action spectra for a wide variety of red algae, however, show marked deviations from their corresponding absorption spectra. The photosynthetic rates are high in the spectral regions absorbed by the water-soluble "phycobilin" pigments (phycoerythrin and phycocyanin), while the light absorbed by chlorophyll and carotenoids is poorly utilized for oxygen production."
Edit: so if the action spectra are to be trusted we need to provide light in the green and understand that it's not benefitting from our normal Blue and Red light scheme.
Your posts make me ponder, a good thing for an aging mind. Thanks!
So I've gotten fortunate and had a few organism I had wanted to sample show up in my systems. Had some Gracilaria pop up in a small test tank.

So I thought I'd revisit the question of red algae.
Here's my absorption spectrum for the gracilaria

Note the phycoerythrin and even a little phycocyanin (confirmed by fluorescence).
This paper provides absorption spectra and action spectra for several red algae and this from porphyrella has an absorption that is a very close match.

But as in the action spectrum Dana posted earlier, Chlorophyll absorbance correlates to low action and is much less of a driver of the action than the phycoerythrin (and phycocyanin).
to quote the paper:
"In green and brown algae, light absorbed by both chlorophyll and carotenoids seems photosynthetically effective, although some inactive absorption by carotenoids is indicated. Action spectra for a wide variety of red algae, however, show marked deviations from their corresponding absorption spectra. The photosynthetic rates are high in the spectral regions absorbed by the water-soluble "phycobilin" pigments (phycoerythrin and phycocyanin), while the light absorbed by chlorophyll and carotenoids is poorly utilized for oxygen production."
Edit: so if the action spectra are to be trusted we need to provide light in the green and understand that it's not benefitting from our normal Blue and Red light scheme.
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
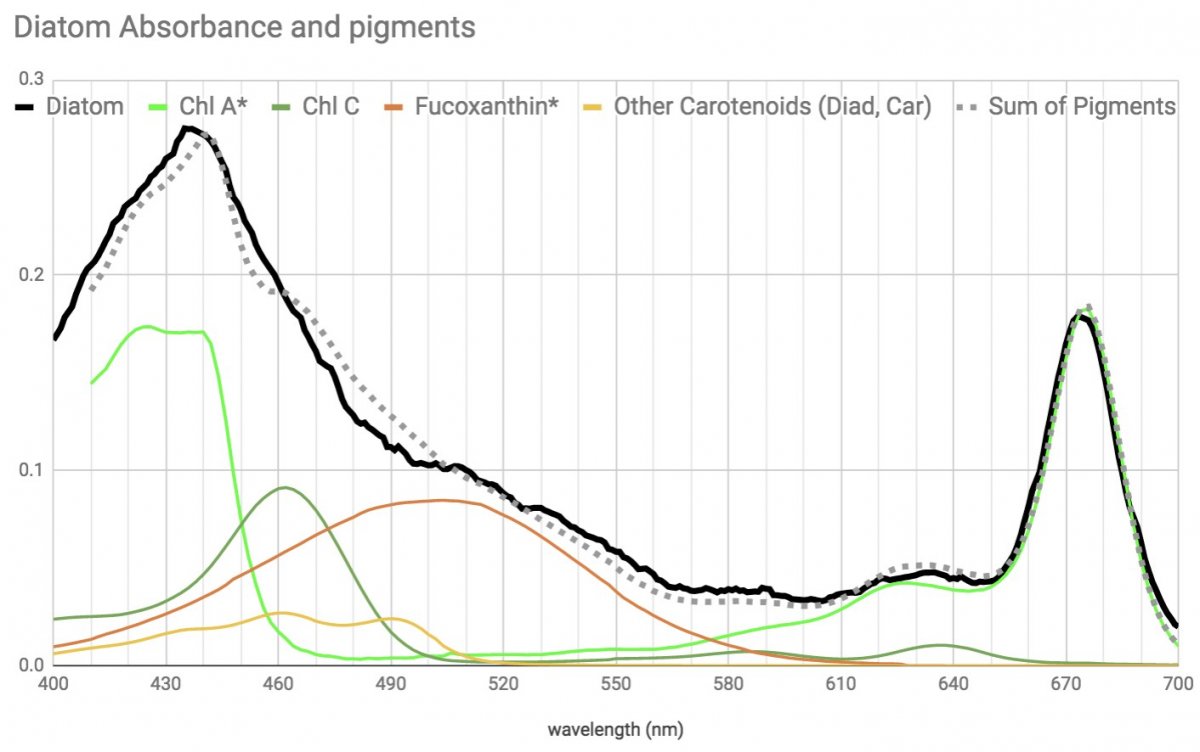
Another organism I had hoped to be able to get and finally cropped up in fairly pure numbers was diatoms.

My expectation was that these would have a pigment profile indistinguishable from zoox since diatoms and dinoflagellates have pigments with the same coverage.
Chl A and Chl C in both, and diatoms Fucoxanthin and dinos Peridinin have almost the same wide absorbance shape with best absorbance from ~450 to ~540 range and both of those helper carotenoids form a unit with Chl A for efficient light harvesting. So really seem like mirror images.
Here's what Diatom absorbance and pigments look like, and below I'll compare to zoox.
and here's zoox harvested from aiptasia
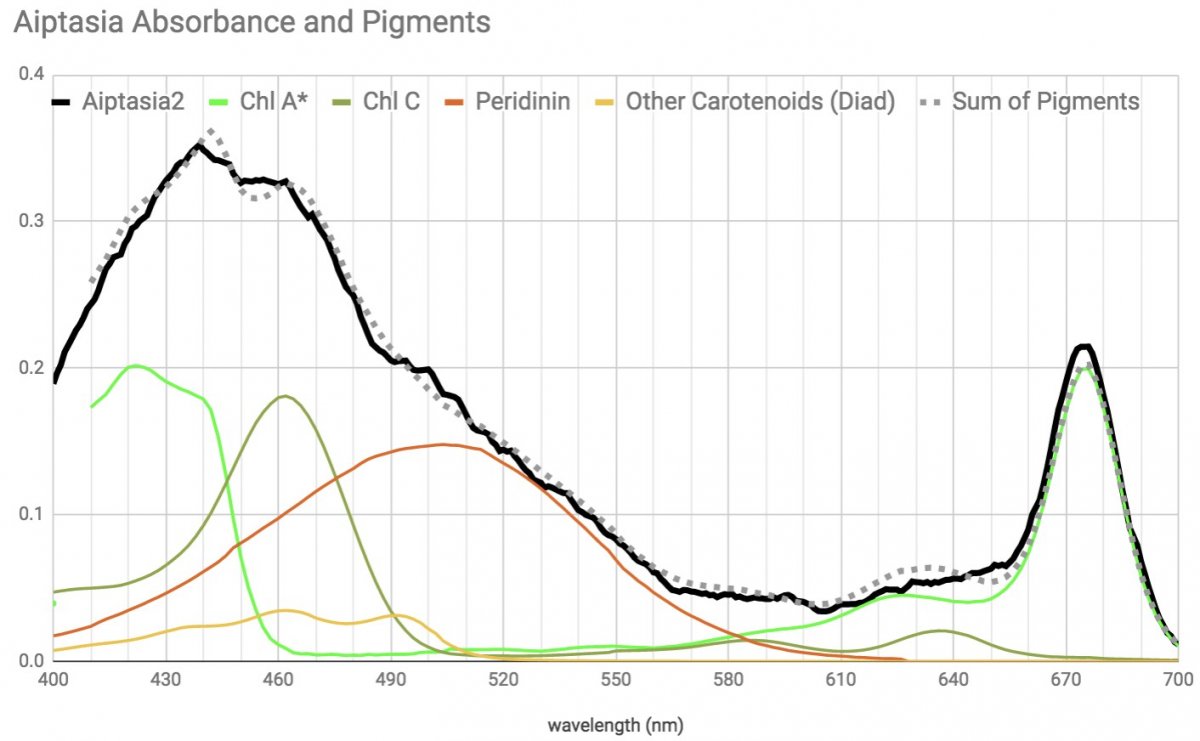
[I chose aipastia as my representative of zoox, because it's the most Chl A / violet absorbance heavy sample I found and is the most representative of the published zoox absorbance in acros and thin branching corals.]
Note that although the diatom has the pigments available to have the same coverage as the zoox, the diatom absorbs relatively less from 450 to 540 (or alternately more in the 400-450).
pure speculation, but I wonder if this may mean there is a relative efficiency difference in the Fucoxanthin-Chlorophyll and Peridinin-Chlorophyll complexes, or if it has to do with more violet light being better for surface growing epiphytes like diatoms vs symbionts growing within an organism. Within the few zoox I've looked at so far, those growing nearer the surface within smaller branchier more transparent organisms have more blue-violet (Chl A) absorbance and those growing within thicker massive type hosts seem to absorb relatively more in the blue-green (Chl C & peridinin) range.
My expectation was that these would have a pigment profile indistinguishable from zoox since diatoms and dinoflagellates have pigments with the same coverage.
Chl A and Chl C in both, and diatoms Fucoxanthin and dinos Peridinin have almost the same wide absorbance shape with best absorbance from ~450 to ~540 range and both of those helper carotenoids form a unit with Chl A for efficient light harvesting. So really seem like mirror images.
Here's what Diatom absorbance and pigments look like, and below I'll compare to zoox.
and here's zoox harvested from aiptasia
[I chose aipastia as my representative of zoox, because it's the most Chl A / violet absorbance heavy sample I found and is the most representative of the published zoox absorbance in acros and thin branching corals.]
Note that although the diatom has the pigments available to have the same coverage as the zoox, the diatom absorbs relatively less from 450 to 540 (or alternately more in the 400-450).
pure speculation, but I wonder if this may mean there is a relative efficiency difference in the Fucoxanthin-Chlorophyll and Peridinin-Chlorophyll complexes, or if it has to do with more violet light being better for surface growing epiphytes like diatoms vs symbionts growing within an organism. Within the few zoox I've looked at so far, those growing nearer the surface within smaller branchier more transparent organisms have more blue-violet (Chl A) absorbance and those growing within thicker massive type hosts seem to absorb relatively more in the blue-green (Chl C & peridinin) range.
- Joined
- Sep 21, 2018
- Messages
- 6,729
- Reaction score
- 7,209
Another organism I had hoped to be able to get and finally cropped up in fairly pure numbers was diatoms.

My expectation was that these would have a pigment profile indistinguishable from zoox since diatoms and dinoflagellates have pigments with the same coverage.
Chl A and Chl C in both, and diatoms Fucoxanthin and dinos Peridinin have almost the same wide absorbance shape with best absorbance from ~450 to ~540 range and both of those helper carotenoids form a unit with Chl A for efficient light harvesting. So really seem like mirror images.
Here's what Diatom absorbance and pigments look like, and below I'll compare to zoox.
and here's zoox harvested from aiptasia

[I chose aipastia as my representative of zoox, because it's the most Chl A / violet absorbance heavy sample I found and is the most representative of the published zoox absorbance in acros and thin branching corals.]
Note that although the diatom has the pigments available to have the same coverage as the zoox, the diatom absorbs relatively less from 450 to 540 (or alternately more in the 400-450).
pure speculation, but I wonder if this may mean there is a relative efficiency difference in the Fucoxanthin-Chlorophyll and Peridinin-Chlorophyll complexes, or if it has to do with more violet light being better for surface growing epiphytes like diatoms vs symbionts growing within an organism. Within the few zoox I've looked at so far, those growing nearer the surface within smaller branchier more transparent organisms have more blue-violet (Chl A) absorbance and those growing within thicker massive type hosts seem to absorb relatively more in the blue-green (Chl C & peridinin) range.
Except for the large size of the 670 nm peak, the spectra resemble a skimmate spectrum
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
Inspired by this thread on using UV glasses to filter out the extreme blue light and thus make coral fluorescence appear brighter, and based on what I've measured in organisms in my tank...
You can do the same with orange/red fluorescence using a Green LED flashlight and a red plastic filter.
Check it out.

What you'll see is corals that fluoresce orange/red, as well as the phycoerythrin in coralline algae and cyano.
(above pic is orange monti cap, some coraline and some cyano)

The different corallines glow slightly different shades.

Zoas in foreground, coralline in background, to the right is cyano.

The cyano is crazy bright, but nothing in my tank glows as bright as this little Acan.
You can do the same with orange/red fluorescence using a Green LED flashlight and a red plastic filter.
Check it out.

What you'll see is corals that fluoresce orange/red, as well as the phycoerythrin in coralline algae and cyano.
(above pic is orange monti cap, some coraline and some cyano)

The different corallines glow slightly different shades.

Zoas in foreground, coralline in background, to the right is cyano.

The cyano is crazy bright, but nothing in my tank glows as bright as this little Acan.
- Joined
- Jun 29, 2017
- Messages
- 532
- Reaction score
- 285
@Dana Riddle - is this being done in a home lab? Do you just have a spec or do you have a complete HPLC-DAD setup?
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
If this is intended for me, most of this is done by a $399 spectrophotometer and materials available in a high school lab.@Dana Riddle - is this being done in a home lab? Do you just have a spec or do you have a complete HPLC-DAD setup?
my device is this 4"x6" cute toy box. It has 405 and 500nm LEDs built-in for fluorescence and an opening where I can shine whatever I have available into the sample chamber and measure emission. Was definitely not intended for what I'm trying to do with it.
Dana has more toys than I do for his explorations. But what exactly, he'll have to say.
- Joined
- Jun 29, 2017
- Messages
- 532
- Reaction score
- 285
Will the company ship it directly to a house?
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
They would.Will the company ship it directly to a house?
I have two Ocean Optics fiber optic spectrometers. One is configured for measuring fluorescence (wide slit) while the other measures up to 1,000nm. As for chemical analyses, I use a Hach colorimeter. No longer have access to an atomic absorption spec, nor a MS/GC.@Dana Riddle - is this being done in a home lab? Do you just have a spec or do you have a complete HPLC-DAD setup?
This is really interesting. Green light as the excitation source. That would have to be a Stokes Shift of at least 50nm, suggesting that there may be several fluorescent proteins acting in relay???
Inspired by this thread on using UV glasses to filter out the extreme blue light and thus make coral fluorescence appear brighter, and based on what I've measured in organisms in my tank...
You can do the same with orange/red fluorescence using a Green LED flashlight and a red plastic filter.
Check it out.

What you'll see is corals that fluoresce orange/red, as well as the phycoerythrin in coralline algae and cyano.
(above pic is orange monti cap, some coraline and some cyano)

The different corallines glow slightly different shades.

Zoas in foreground, coralline in background, to the right is cyano.

The cyano is crazy bright, but nothing in my tank glows as bright as this little Acan.
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
I had looked at fluorescence from this stuff several times and was assuming it was just phycoerythrin absorbing green and emitting with a peek at 575 sliding into the orange that was getting through the red filter.This is really interesting. Green light as the excitation source. That would have to be a Stokes Shift of at least 50nm, suggesting that there may be several fluorescent proteins acting in relay???
But just to check your hunch, I excited whole live cyano cells instead of the extracted pigments that I had always done before. And wow! It's as weird as you suspected. Will post details later.
- Joined
- May 22, 2016
- Messages
- 6,588
- Reaction score
- 10,170
there may be several fluorescent proteins acting in relay???
But just to check your hunch, I excited whole live cyano cells instead of the extracted pigments that I had always done before. And wow! It's as weird as you suspected. Will post details later.
Here's what I got when I looked at live red cyano cells excited by green LED flashlight.
From this paper: Adventures with Cyanobacteria
"...If we excite D [Donor] and all fluorescence appears from A [Acceptor], we know that there has been 100% energy transfer from D to A. However, if fluorescence appears from both D and A, we know that there has been a partial energy transfer... Duysens (1952) showed efficient energy transfer from phycobilins to Chl a in the cyanobacterium Oscillatoria."
"...phycocyanin (with absorption bands at 580, 625, and 634–637 nm) and allophycocyanin (at 650 nm) are responsible for the broad fluorescence band at 653–655 nm; Chl a 670 is responsible for fluorescence band at 680 nm (F680), and Chl a 678 is responsible for fluorescence band at 685 nm (F685)..."
and here's an illustration from the same paper showing the scheme.
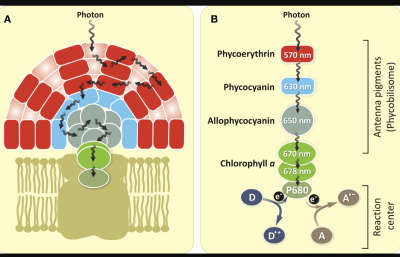
Coralline algae possesses the same pigments so likely is playing a similar game.
The Acan legitimately has a long stokes shift with pigments that fluoresce deep orange when excited at a range of wavelenghts from violet to green. It seems likely to be a "kaede" type pigment as @Dana Riddle described in this article.
I may also try to do a live fluorescence of the monti cap and see if there's something I missed. All I got on previous looks was an orange pigment peaking at 575 nm emission.
Similar threads
- Replies
- 3
- Views
- 456



















