I'm unsure of the spectrometer you're using. Is its slit suitable for work with fluorescence? Can it correct for electrical dark and have wavelength and boxcar averaging? Signals can get noisy sometimes. Just some thoughts on the instrument itself - I suspect you are correct in your analyses but I want to ask just to make sure. I'd have to look at MAA absorption spectra - perhaps you already have. I don't think there is a UV protectant with a bandwidth that extends into the visible spectrum but then its been a while since I've looked into these. As for NowGFP, I seem to recall that it is a construct from A. victoria and highly sensitive to pH, but that is not to say that it does not occur naturally but is yet undescribed.So here's my first attempt at isolating pigments from a coral and not the zoox. (After that I can subtract those pigments and look just at the zoox absorbance in a coral.)
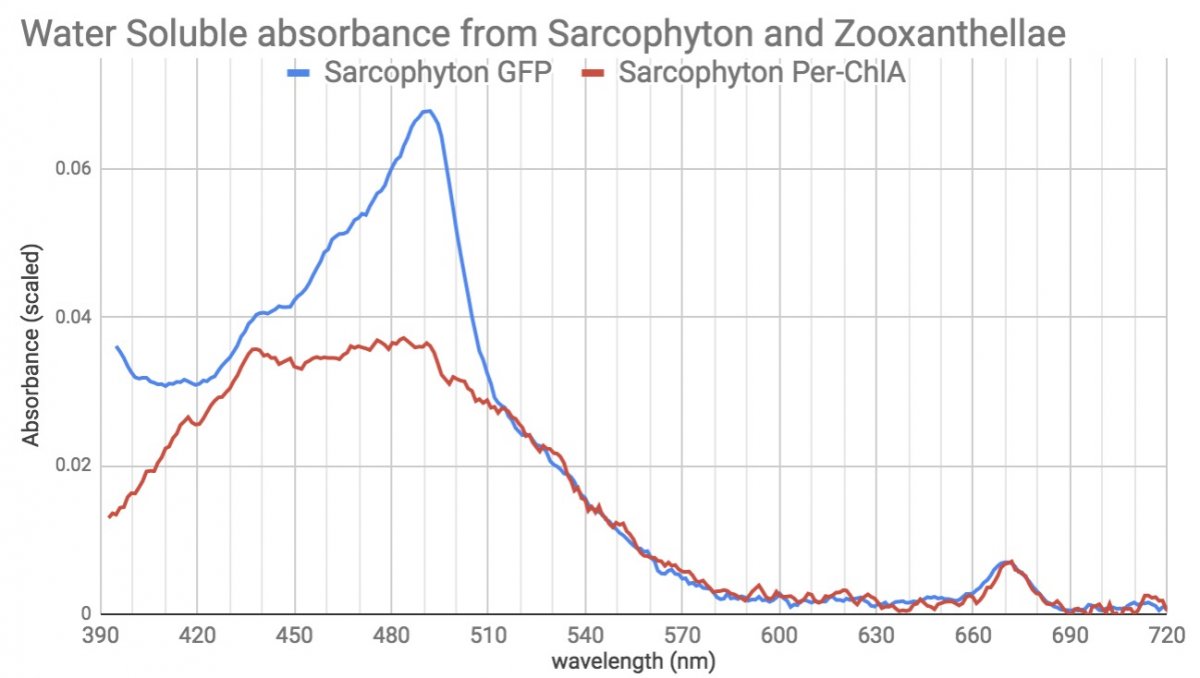
In blue is what dissolved out of Neon Sarcophyton tissue, and in red is what dissolved out of the zoox in the coral. The tissue solution is dominated by a green fluorescent protein, and the red line is almost a pure Peridinin-Chlorophyll protein (spectrum for comparison). You can see each has a little contamination from the other in it.
But subtract them and what you're left with is this profile of a Green Fluorescent Protein...(with scaled Fluorescence shown)
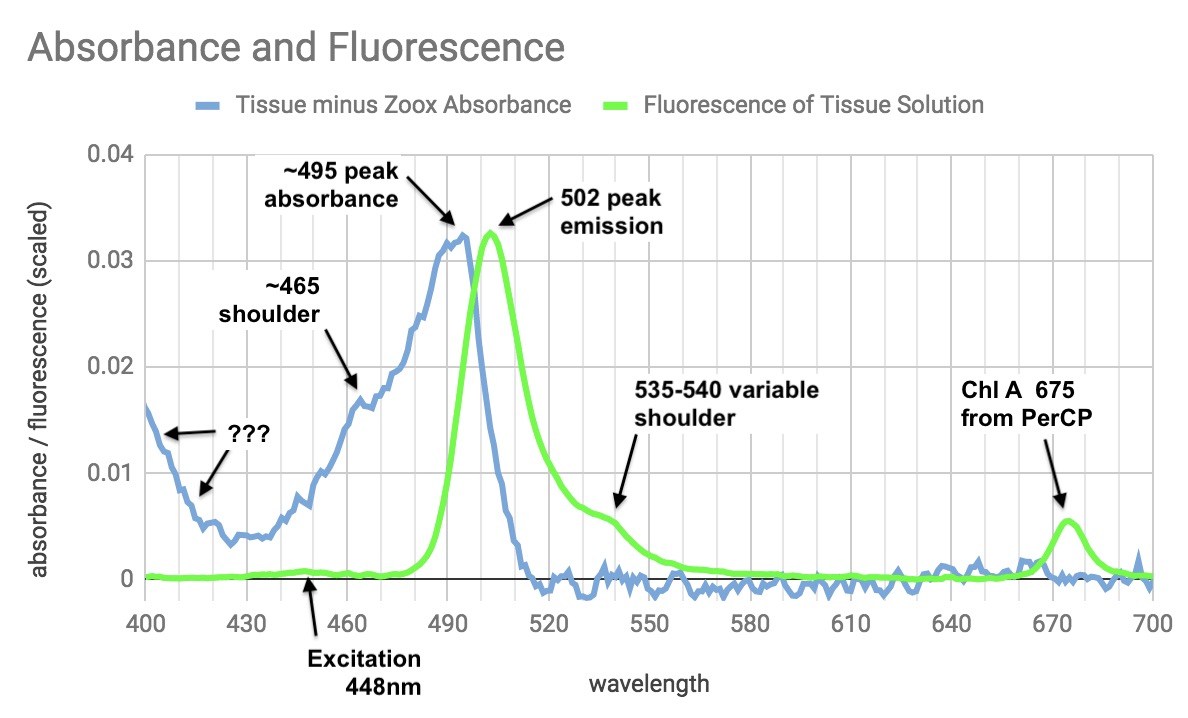
This is the "neon" in my neon green sarcophyton. Ignoring the ChlA fluorescence, the absorbance/emission sounds kind of like P503 from a carpet anemone in Dana's article, though there are many similar GFPs. It also looks a lot like this stupidly named NowGFP.
(@Dana Riddle What bugs me is the increased absorbance going from 420 to 400nm (I don't trust my stuff below 400nm). The absorbance increase there is probably real - I've seen it in different solutions out of the sarcophyton tissue done different ways. But it doesn't seem connected to fluorescence. There is fluorescence at 502 & 675 in the sample when excited with 405nm, but it's quite weak - much weaker than at 430, etc.
Makes me think most of the 400nm absorbance is an unrelated nonfluorescent pigment. Weird.)
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
A Spectrophotometer view of Photosynthetic Organisms in our Tanks
- Thread starter taricha
- Start date
- Tagged users None
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
Yeah, after looking up lab grade devices and discussions on slits for fluorescence electrical dark etc, I'm going to say no.I'm unsure of the spectrometer you're using. Is its slit suitable for work with fluorescence? Can it correct for electrical dark and have wavelength and boxcar averaging?...
I'd have to look at MAA absorption spectra - perhaps you already have. I don't think there is a UV protectant with a bandwidth that extends into the visible spectrum but then its been a while since I've looked into these. As for NowGFP, I seem to recall that it is a construct from A. victoria and highly sensitive to pH, but that is not to say that it does not occur naturally but is yet undescribed.
my device is this 4"x6" cute toy box. It has 405 and 500nm LEDs built-in for fluorescence and an opening where I can shine whatever I have available into the sample chamber and measure emission. Was definitely not intended for what I'm trying to do with it.
on the NowGFP, I saw "derived from A. victoria", and missed the part that it was several artificial steps removed from the natural GFP produced by the anemone.
Thanks for tip on MAAs, but unless there's a bunch of cyano making scytonemin in there... no luck (absorption spectra of MAAs).
I tried looking up which pigments are responsible for the yellow of sarcophyton elegans, but haven't found anything helpful yet.
Last edited:
Goodness, a spec for $399! Makes me wonder how Ocean Optics stays in business. They're getting close to 3 grand for theirs. Glad I bought mine when they were *reasonable* (ha!) I applaud your ingenuity in making the spec work for you. I threw the PAM fluorometer instruction book out the window in order to look at photosynthesis when exposed to different aquarium light sources. Re: the Sarcophyton elegans yellow coloration. If I recall correctly, that's a chromoprotein (non-fluorescent) and its not common. If my failing memory serves, I've seen it only in another coral (a Hawaiian Porites specimen.)Yeah, after looking up lab grade devices and discussions on slits for fluorescence electrical dark etc, I'm going to say no.
my device is this 4"x6" cute toy box. It has 405 and 500nm LEDs built-in for fluorescence and an opening where I can shine whatever I have available into the sample chamber and measure emission. Was definitely not intended for what I'm trying to do with it.
on the NowGFP, I saw "derived from A. victoria", and missed the part that it was several artificial steps removed from the natural GFP produced by the anemone.
Thanks for tip on MAAs, but unless there's a bunch of cyano making scytonemin in there... no luck (absorption spectra of MAAs).
I tried looking up which pigments are responsible for the yellow of sarcophyton elegans, but haven't found anything helpful yet.
Keep up the good work!
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
So I took another stab at it, and cleaned my samples up to try to increase the signal to noise ratio.
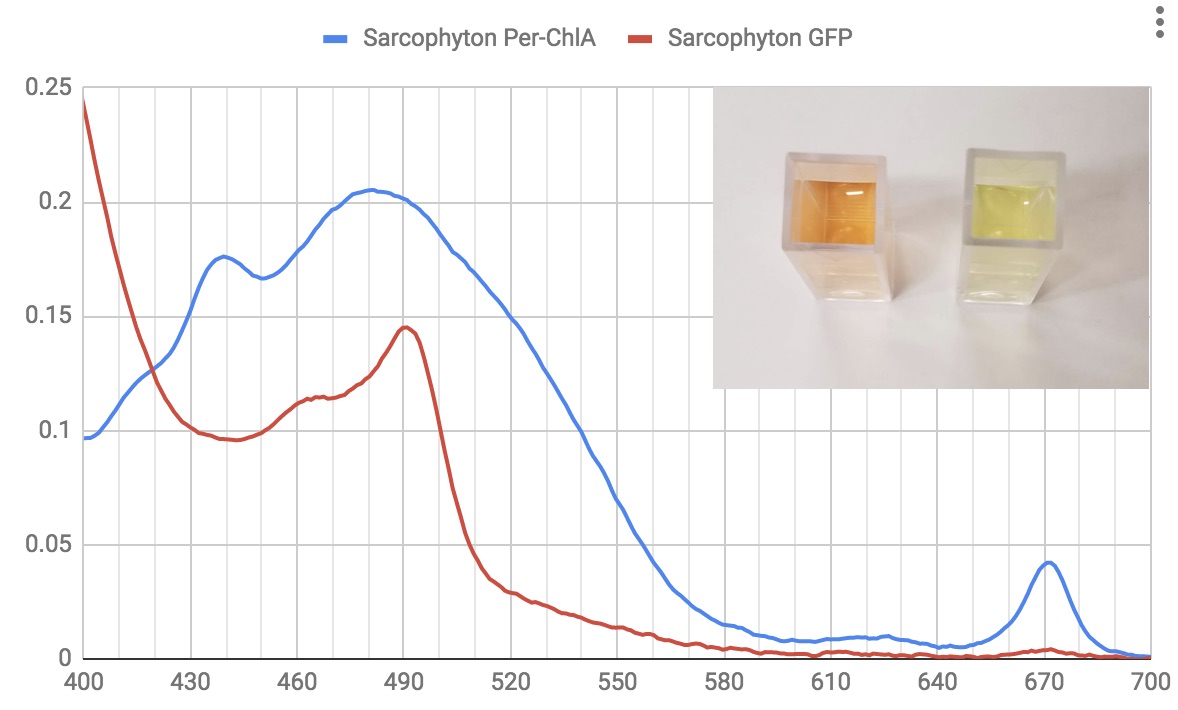
Water soluble solutions: Blue line is from sarcophyton zoox, red from the sarcophyton tissue. Actual solutions in inset picture.
Subtracting and correcting for the smaller but still present PerCP in the tissue sample, and plotting fluorescence of the tissue sample...
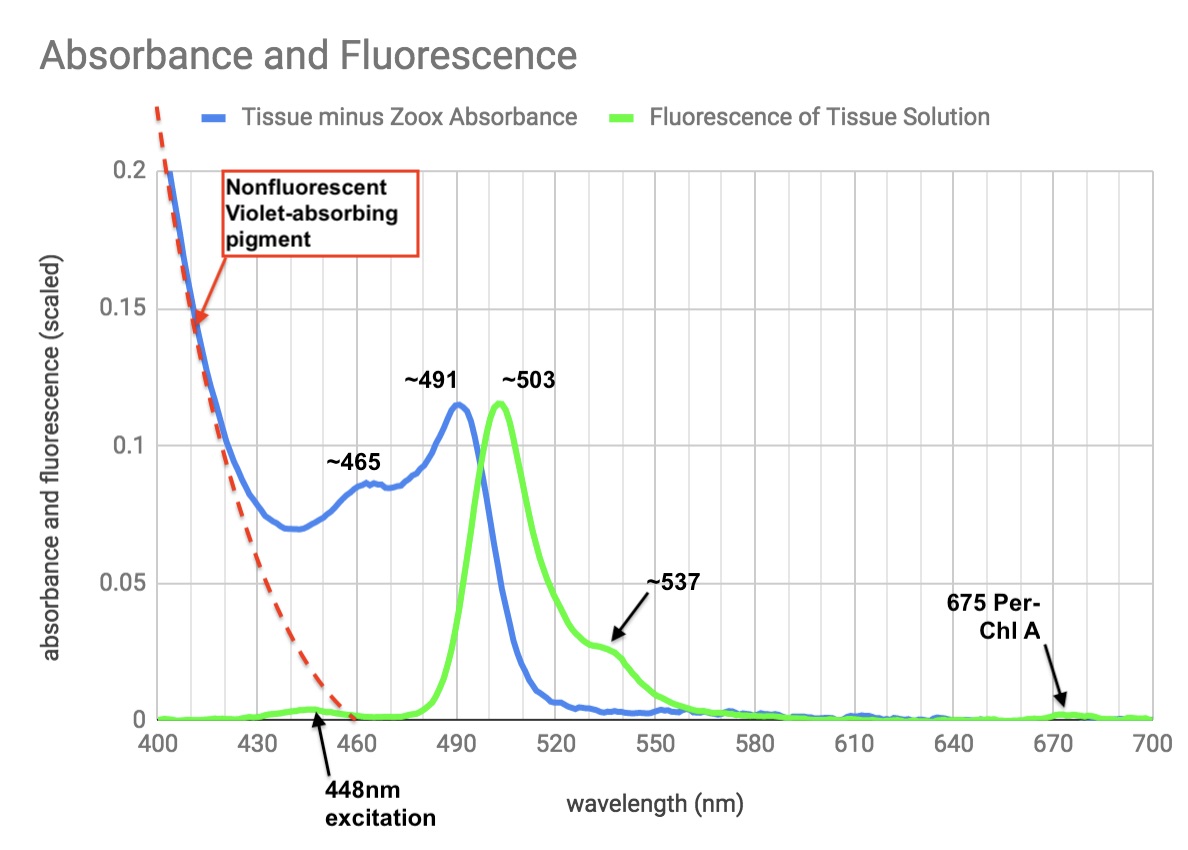
Peaks, shoulders and excitation wavelength marked on plot.
Here's what I feel confident about the weird violet absorbance, that makes the solution look yellow.
Water soluble solutions: Blue line is from sarcophyton zoox, red from the sarcophyton tissue. Actual solutions in inset picture.
Subtracting and correcting for the smaller but still present PerCP in the tissue sample, and plotting fluorescence of the tissue sample...
Peaks, shoulders and excitation wavelength marked on plot.
Here's what I feel confident about the weird violet absorbance, that makes the solution look yellow.
- It's not related to the Green Fluorescent Protein - it shows up in different ratios to the GFP.
- Fluorescent response of GFP gets steadily worse as excitation moves from 500->448->430->405->Blacklight Fluorescent. Fluorescence virtually undetectable in response to blacklight (~370nm).
- It's actual absorbance and not just scattering of violet light.
I'll check my database and see if I have any info on a yellow chromoprotein. Tomorrow. I'm beat.So I took another stab at it, and cleaned my samples up to try to increase the signal to noise ratio.
Water soluble solutions: Blue line is from sarcophyton zoox, red from the sarcophyton tissue. Actual solutions in inset picture.

Subtracting and correcting for the smaller but still present PerCP in the tissue sample, and plotting fluorescence of the tissue sample...

Peaks, shoulders and excitation wavelength marked on plot.
Here's what I feel confident about the weird violet absorbance, that makes the solution look yellow.
@Dana Riddle I don't know how that yellow non-fluorescent chromoprotein from s. elegans is supposed to look like or behave, but in my head I imagine it's somewhat like that. Maybe the neon sarcophyton has something related.
- It's not related to the Green Fluorescent Protein - it shows up in different ratios to the GFP.
- Fluorescent response of GFP gets steadily worse as excitation moves from 500->448->430->405->Blacklight Fluorescent. Fluorescence virtually undetectable in response to blacklight (~370nm).
- It's actual absorbance and not just scattering of violet light.
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
Good news is that at first look, my orange monti cap has been better behaved. So far only major coral pigment seems to match pigment profile from an orange monti digi in Dana's article.
So far I'm bad at guessing what will be easy and what will be a challenge
So far I'm bad at guessing what will be easy and what will be a challenge
Here's the absorbance of a yellow chromoprotein from an Acropora specimen - this is the only yellow CP I have in the database. Have no idea if it is exactly the same as that seen in S. elegans. This is from Shibata, 1969.
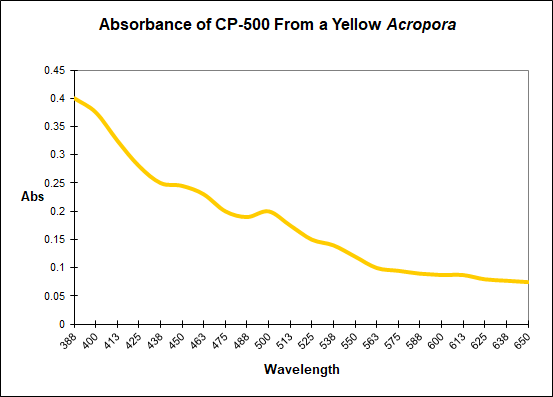
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
Took me quite some time to work out how to isolate zoox out of a stony coral, but here we go.
I plan to do two Monti caps: orange and green(w/red polyps), two monti digi: "purple" and green, a green candy cane coral, and maybe one other LPS.
This is the zoox spectrum from an Orange/Red Monti Cap (followed by the spectrum of pigments from coral tissue.)
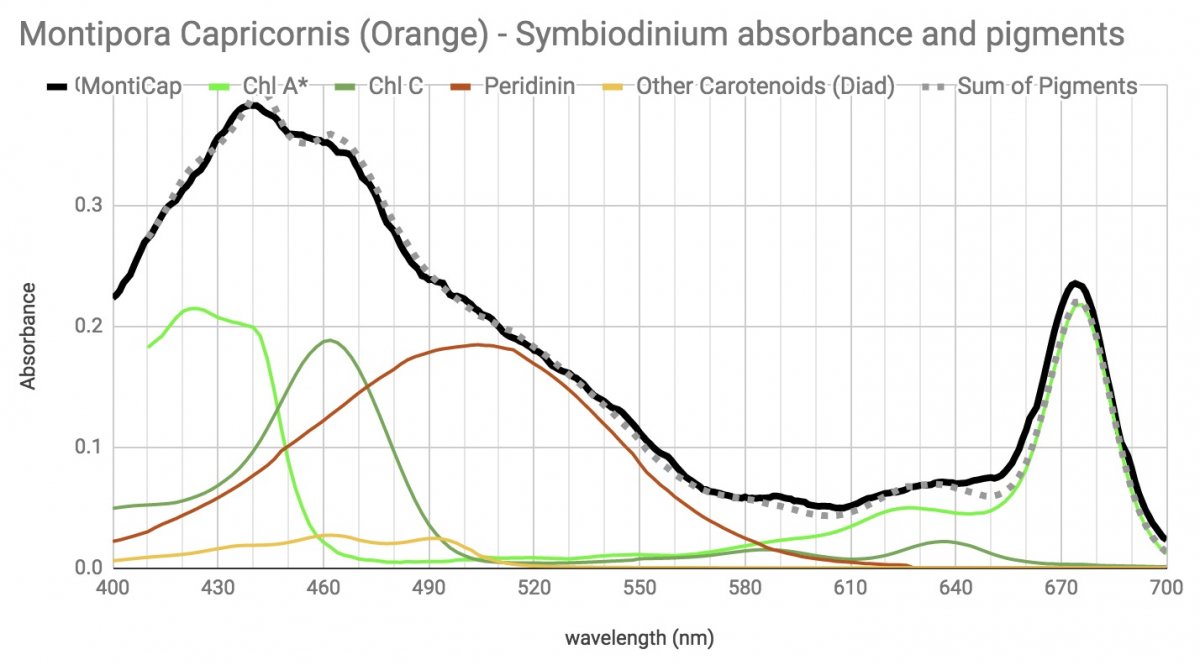
I think I expected more photoprotective pigments because it's got quite a deep orange/red/brown color to it. Very high in tank close to lights, and gets a couple of hours of direct sun as well.
I also keep looking at how weirdly similar it is to the zoox from the Aiptasia tentacles. About as identical as you can get, didn't expect that either.
And here's the pigments absorption/emission from the coral tissue itself - zoox pigments excluded.
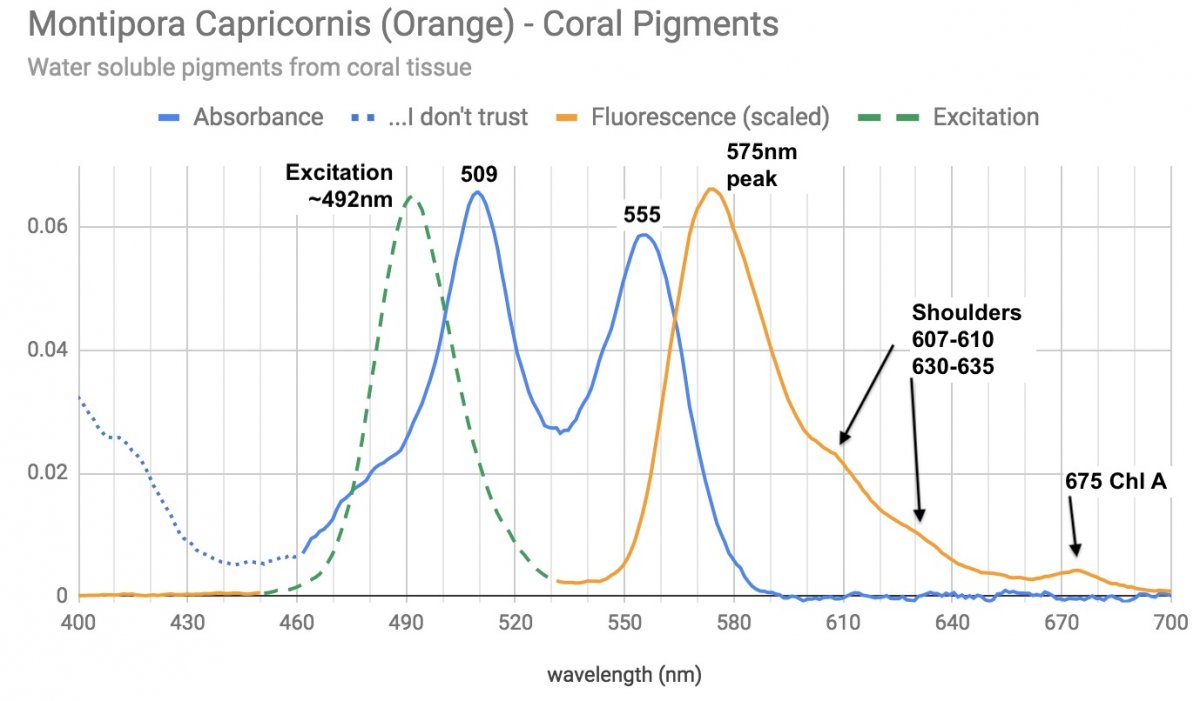 Looks similar to a version of P-575 (from an orange monti digi) in Dana's article.
Looks similar to a version of P-575 (from an orange monti digi) in Dana's article.
double barrel absorption peaks around ~509 and ~555, strong fluorescent emission at 575 - with plenty of light emitted in the yellow,orange, red all the way down to the ChlA fluorescence from a little contamination.
[the violet region may well be just artifacts and noise I'm having some difficulty preventing/removing. It's certainly not fluorescent, or related to the main pigments.]
Next up will be the monti cap that's as green as the last one was orange (and a few red polyps thrown in there).
I plan to do two Monti caps: orange and green(w/red polyps), two monti digi: "purple" and green, a green candy cane coral, and maybe one other LPS.
This is the zoox spectrum from an Orange/Red Monti Cap (followed by the spectrum of pigments from coral tissue.)
I think I expected more photoprotective pigments because it's got quite a deep orange/red/brown color to it. Very high in tank close to lights, and gets a couple of hours of direct sun as well.
I also keep looking at how weirdly similar it is to the zoox from the Aiptasia tentacles. About as identical as you can get, didn't expect that either.
And here's the pigments absorption/emission from the coral tissue itself - zoox pigments excluded.
double barrel absorption peaks around ~509 and ~555, strong fluorescent emission at 575 - with plenty of light emitted in the yellow,orange, red all the way down to the ChlA fluorescence from a little contamination.
[the violet region may well be just artifacts and noise I'm having some difficulty preventing/removing. It's certainly not fluorescent, or related to the main pigments.]
Next up will be the monti cap that's as green as the last one was orange (and a few red polyps thrown in there).
Some time ago I had a SPS tank. When switching between 420, 455, 490nm LEDs some thing happened that at the time I put down to coincidence, but has always played on my mind.
The two 'hardy' corals, similar to monti cap and green slimmer, both green variants, were most fluorescent between 460 - 490. At 490 all other sps (40+) appeared generally blue-grey, except those two. All the other corals looked best around 420-450.
The two 'hardy' corals, similar to monti cap and green slimmer, both green variants, were most fluorescent between 460 - 490. At 490 all other sps (40+) appeared generally blue-grey, except those two. All the other corals looked best around 420-450.
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
Very interesting comment and been thinking about it as I look at a couple of other corals.Some time ago I had a SPS tank. When switching between 420, 455, 490nm LEDs some thing happened that at the time I put down to coincidence, but has always played on my mind.
The two 'hardy' corals, similar to monti cap and green slimmer, both green variants, were most fluorescent between 460 - 490. At 490 all other sps (40+) appeared generally blue-grey, except those two. All the other corals looked best around 420-450.
The green from sarcophyton and green from montipora have more difference than I expected. Partially in the kind of green they fluoresce, but there's also a big difference in the excitation wavelength. The monti fluorescence responds to shorter wavelength light than the sarco does.
Last edited:
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
As I've been looking at coral host pigments vs zooxanthellae pigments, it's been very surprising. The photopigments in zoox cells are all the same pigments occasionally in different ratios. But the fluorescent coral pigments are endlessly different.
I have looked at several green fluorescent corals that I expected to have essentially the same green fluorescent proteins. Nope. Totally different emission spectra, and even their absorption spectra differs greatly in what light drives their fluorescence.
I have looked at several green fluorescent corals that I expected to have essentially the same green fluorescent proteins. Nope. Totally different emission spectra, and even their absorption spectra differs greatly in what light drives their fluorescence.
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
finding Chlorophyll A, B, and C in skimmer sludge.
I apologize for this digression since it's kind of off-topic for this forum, but it's on-topic for this weird thread. [Next post I'll get more on-topic with a comparison of green fluorescence in a couple of different corals ]
]
I didn't realize that Chlorophyll A, B, and C can be separately excited and their fluorescence distinguished.
While trying to prove what class a mystery aquarium pest belongs to I stumbled across this.
Here's extracted samples of Derbesia (Chl A & B) and Zooxanthellae from Aiptasia (Chl A & C), excited with different LED wavelengths.
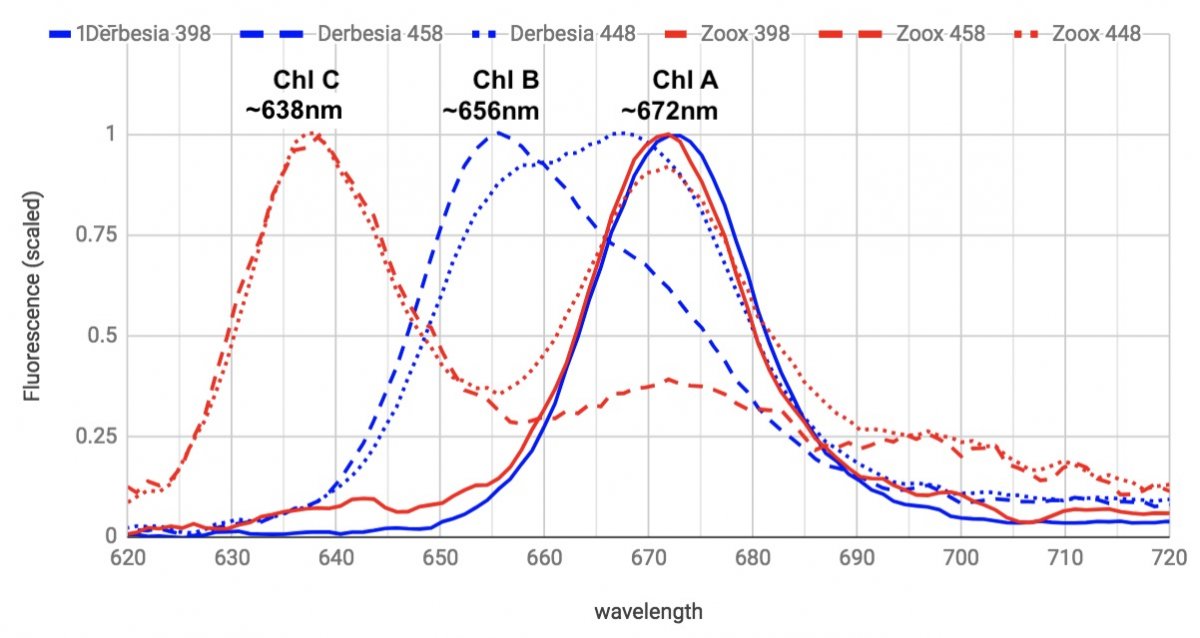
The 398nm LED excites pretty much just Chl A, and the 458nm excites B & C well, while muting the Chl A effect. 448nm LED excites both and produces a combined A/B peak, or a A/C double peak.
Now, here's what I think is nifty.
Sludge from my skimmer neck actually has plenty of Chlorophyll and related breakdown products. All 3 Chlorophylls are detectable.
Chl A (cyano & others) dominates, Chl B (derbesia in tank and chaeto in sump) is present and clear once A is subtracted out, and C (diatoms and dinoflagellates) is barely but definitely there.
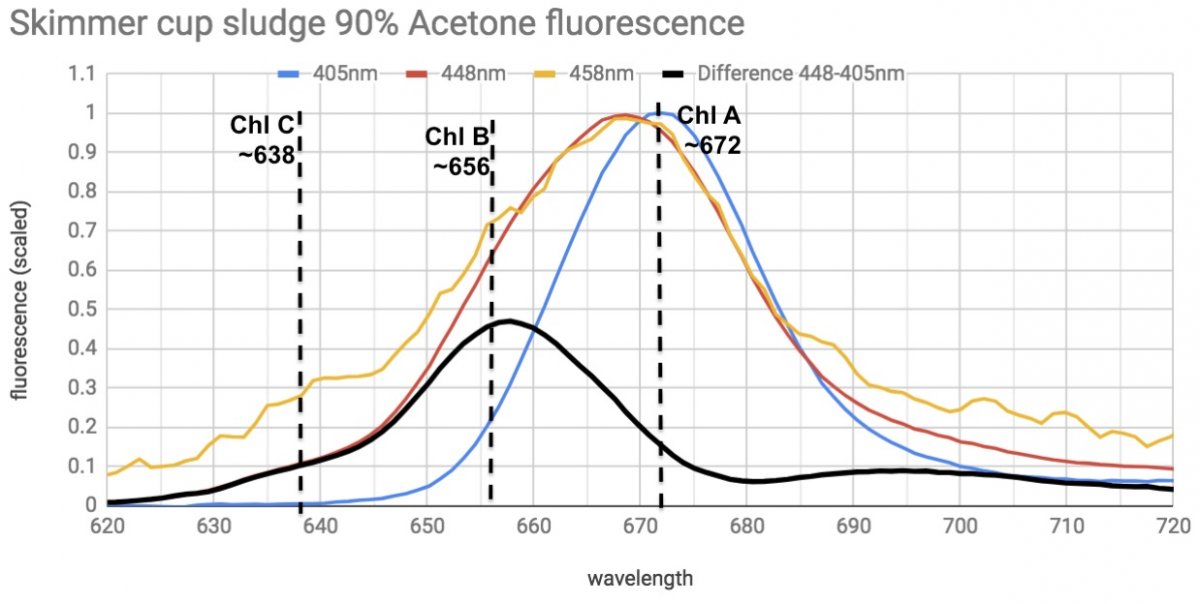
The black line is the fluorescence excited by 448nm LED with the Chl A signal from the 405nm LED subtracted out, leaving Chl B and a little C.
So it's a little peek into the origin of stuff that ends up in my skimmer.
I apologize for this digression since it's kind of off-topic for this forum, but it's on-topic for this weird thread. [Next post I'll get more on-topic with a comparison of green fluorescence in a couple of different corals
I didn't realize that Chlorophyll A, B, and C can be separately excited and their fluorescence distinguished.
While trying to prove what class a mystery aquarium pest belongs to I stumbled across this.
Here's extracted samples of Derbesia (Chl A & B) and Zooxanthellae from Aiptasia (Chl A & C), excited with different LED wavelengths.
The 398nm LED excites pretty much just Chl A, and the 458nm excites B & C well, while muting the Chl A effect. 448nm LED excites both and produces a combined A/B peak, or a A/C double peak.
Now, here's what I think is nifty.
Sludge from my skimmer neck actually has plenty of Chlorophyll and related breakdown products. All 3 Chlorophylls are detectable.
Chl A (cyano & others) dominates, Chl B (derbesia in tank and chaeto in sump) is present and clear once A is subtracted out, and C (diatoms and dinoflagellates) is barely but definitely there.
The black line is the fluorescence excited by 448nm LED with the Chl A signal from the 405nm LED subtracted out, leaving Chl B and a little C.
So it's a little peek into the origin of stuff that ends up in my skimmer.
- Joined
- Sep 18, 2017
- Messages
- 5,627
- Reaction score
- 3,458
https://www.advancedaquarist.com/2012/10/aafeature_album/image014.jpg/image_fullAs I've been looking at coral host pigments vs zooxanthellae pigments, it's been very surprising. The photopigments in zoox cells are all the same pigments occasionally in different ratios. But the fluorescent coral pigments are endlessly different.
I have looked at several green fluorescent corals that I expected to have essentially the same green fluorescent proteins. Nope. Totally different emission spectra, and even their absorption spectra differs greatly in what light drives their fluorescence.
- Joined
- Sep 21, 2018
- Messages
- 6,725
- Reaction score
- 7,203
finding Chlorophyll A, B, and C in skimmer sludge.
I apologize for this digression since it's kind of off-topic for this forum, but it's on-topic for this weird thread. [Next post I'll get more on-topic with a comparison of green fluorescence in a couple of different corals]
I didn't realize that Chlorophyll A, B, and C can be separately excited and their fluorescence distinguished.
While trying to prove what class a mystery aquarium pest belongs to I stumbled across this.
Here's extracted samples of Derbesia (Chl A & B) and Zooxanthellae from Aiptasia (Chl A & C), excited with different LED wavelengths.

The 398nm LED excites pretty much just Chl A, and the 458nm excites B & C well, while muting the Chl A effect. 448nm LED excites both and produces a combined A/B peak, or a A/C double peak.
Now, here's what I think is nifty.
Sludge from my skimmer neck actually has plenty of Chlorophyll and related breakdown products. All 3 Chlorophylls are detectable.
Chl A (cyano & others) dominates, Chl B (derbesia in tank and chaeto in sump) is present and clear once A is subtracted out, and C (diatoms and dinoflagellates) is barely but definitely there.

The black line is the fluorescence excited by 448nm LED with the Chl A signal from the 405nm LED subtracted out, leaving Chl B and a little C.
So it's a little peek into the origin of stuff that ends up in my skimmer.
Nice demonstration!
Doing this sort of analysis on “fresh” sludge (centrifuged fresh skimmate) sampled throughout the day or week might be an approach to trend the type of stuff in an aquarium water. Might it predict the onset of a dinoflagellate or cyanobacteria bloom, a rise or fall in total organic carbon?
On another topic, and totally way off topic, is there an inexpensive way to make a flow cell to continuously monitor the visible spectrum or single wavelength of skimmate? Applying what you demonstrated on a continuous basis might be incredibly interesting.
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
Oooh. That's awesome! I'll reference that a lot.
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
Nice demonstration!
Doing this sort of analysis on “fresh” sludge (centrifuged fresh skimmate) sampled throughout the day or week might be an approach to trend the type of stuff in an aquarium water. Might it predict the onset of a dinoflagellate or cyanobacteria bloom, a rise or fall in total organic carbon?
On another topic, and totally way off topic, is there an inexpensive way to make a flow cell to continuously monitor the visible spectrum or single wavelength of skimmate? Applying what you demonstrated on a continuous basis might be incredibly interesting.
Cyano is easy. Could detect even tiny amounts. You've got two bright Fluorescent targets, Chl A and Phycoerythrin. Finding both is a dead giveaway.
Dinos... maybe. Lemme leave it there for now.
And on the 2nd idea, now you're gonna make me read how a flow cytometer works.
Q is what can be detected "live" versus doing extractions. I think the organics would block a lot of interesting wavelengths.
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
Next post I'll get more on-topic with a comparison of green fluorescence in a couple of different corals
Here's 5 different green coral fluorescent pigment profiles. Though they might all look similarly green, there's a good bit of variation in the details of their behavior both in the light they emit, and in the excitation wavelengths that drive them.
The 5 green corals I sampled here are Neon Green Sarcophyton, Caulastrea Candy Cane, a green Monti Cap with red polyps, A solid green Monti Digitata, and finally a Green star polyp. Lets go from simplest, to the ones with most going on.
First up, sarcophyton.
This absorbs strongly in the mid to high 400nm range and has the same emission spectrum with a peak at 503 and a shoulder at 537 regardless of the light used to excite it. Note that it almost totally unresponsive to extreme violet/UV. The peak and shoulder features seen here show up in a lot of pigments.
Next, Caulastrea (Candy Cane).
This one also has the same fluorescent emission pattern with a wide peak around 476 regardless of the light driving it. Though the peak is technically in the Teal range, it's so wide it gives off plenty of green as well. This pigment absorbs broadly and strongly in the violet and had the strongest UV response of anything I saw.
3rd and 4th, Montiporas.
The green pigment here absorbs well from the extreme violet/UV up to the mid 400s, and emits green with the peak and shoulder feature again. There is a second absorbance that peaks around 560 but absorbs in the low 500s too that appears connected to the red fluorescence in the polyps. There's a possible orange emission around 575 (which is the dominant emission in the orange monti cap) and a strong emission at 675 where Chlorophyll fluoresces. See here for Dana's discussion(article) of a pocillopora damicornis emitting(pic) red in similar way.
Montipora Digitata.
This solid green monti digi has a double peak or more likely, is two pigments. Excitation light at 458nm (and everything shorter wavelength than that) excites the familiar peak and shoulder emission pattern. But excitation at 492 - basically on the left absorbance peak, excites the main emission at 517 narrowly and strongly, but the shoulder at 553 disappears. This is another green pigment(s) that is almost totally unresponsive to extreme violet/UV.
Green Star Polyp.
The weediest coral has the weirdest green fluorescent complex that I saw.
Possibly something like that may be happening here? The main emission peak (520) and shoulder (554) can be excited by light at the left absorbance (450) or the right absorption (507). But the left absorbance peak also generates light with a peak around 480, and that in turn would also excite the right absorbance peak.It would be interesting to use various LEDs to excite the pigments to see if pigments are relaying fluorescence - in other words, is the florescence of one protein used as the excitation source for another protein.
(Through this, I've been thinking about what actually makes corals "pop," and how to quantify and evaluate the best light to accentuate particular coral pigments. But that discussion is for another post.)
- Joined
- Sep 21, 2018
- Messages
- 6,725
- Reaction score
- 7,203
Here's 5 different green coral fluorescent pigment profiles. Though they might all look similarly green, there's a good bit of variation in the details of their behavior both in the light they emit, and in the excitation wavelengths that drive them.
The 5 green corals I sampled here are Neon Green Sarcophyton, Caulastrea Candy Cane, a green Monti Cap with red polyps, A solid green Monti Digitata, and finally a Green star polyp. Lets go from simplest, to the ones with most going on.
First up, sarcophyton.
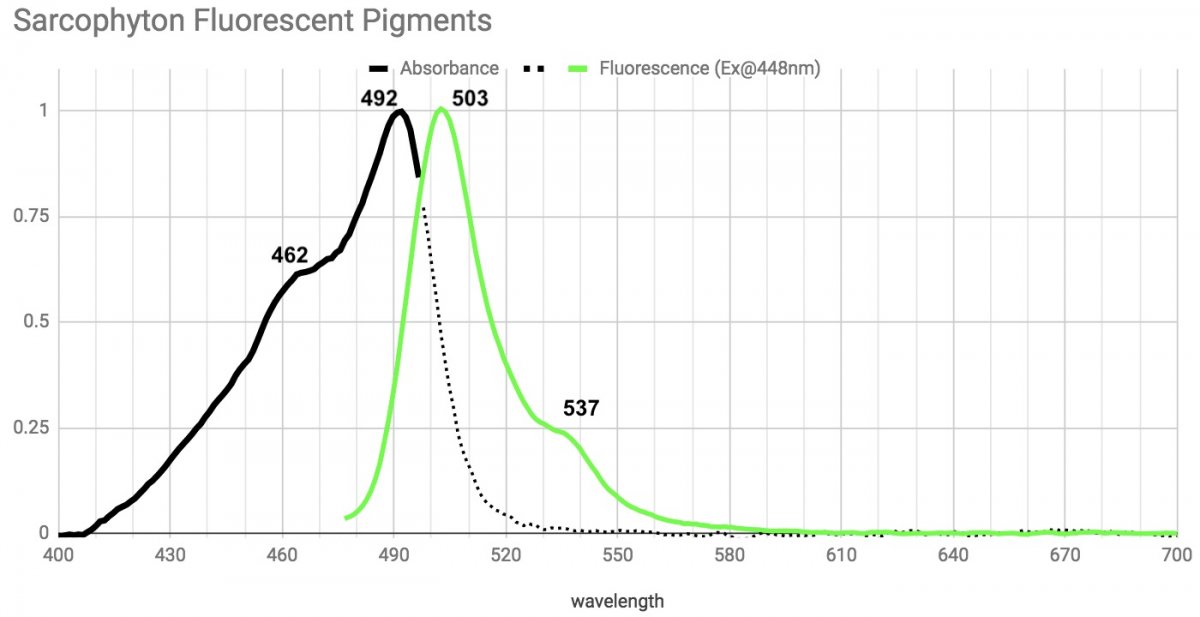
This absorbs strongly in the mid to high 400nm range and has the same emission spectrum with a peak at 503 and a shoulder at 537 regardless of the light used to excite it. Note that it almost totally unresponsive to extreme violet/UV. The peak and shoulder features seen here show up in a lot of pigments.
Next, Caulastrea (Candy Cane).
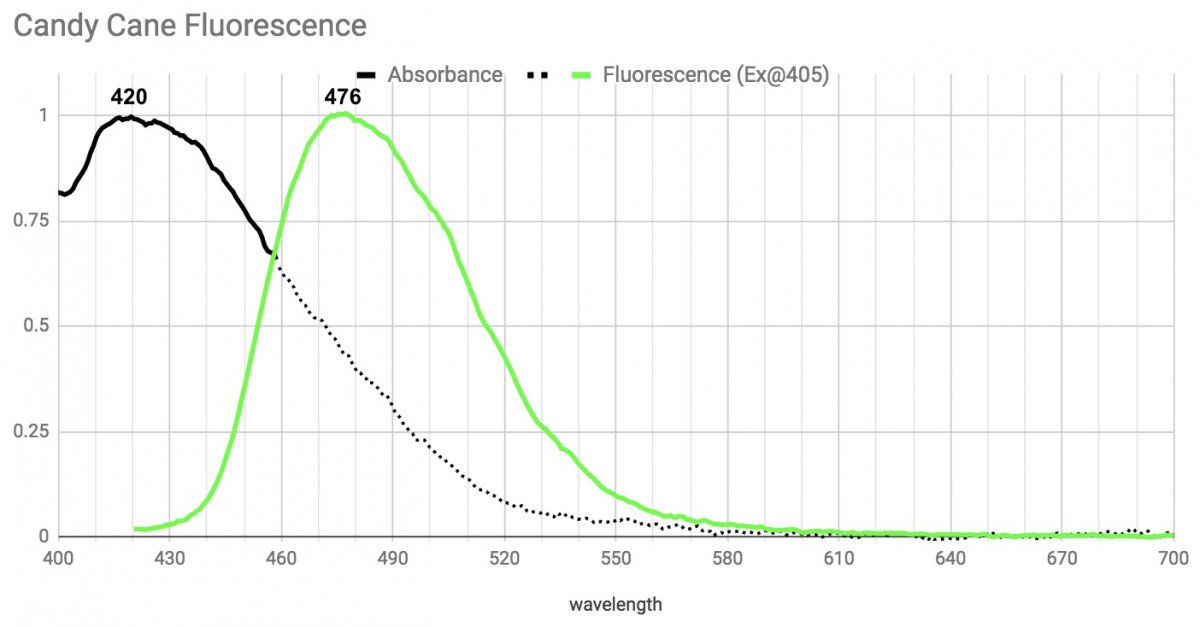
This one also has the same fluorescent emission pattern with a wide peak around 476 regardless of the light driving it. Though the peak is technically in the Teal range, it's so wide it gives off plenty of green as well. This pigment absorbs broadly and strongly in the violet and had the strongest UV response of anything I saw.
3rd and 4th, Montiporas.
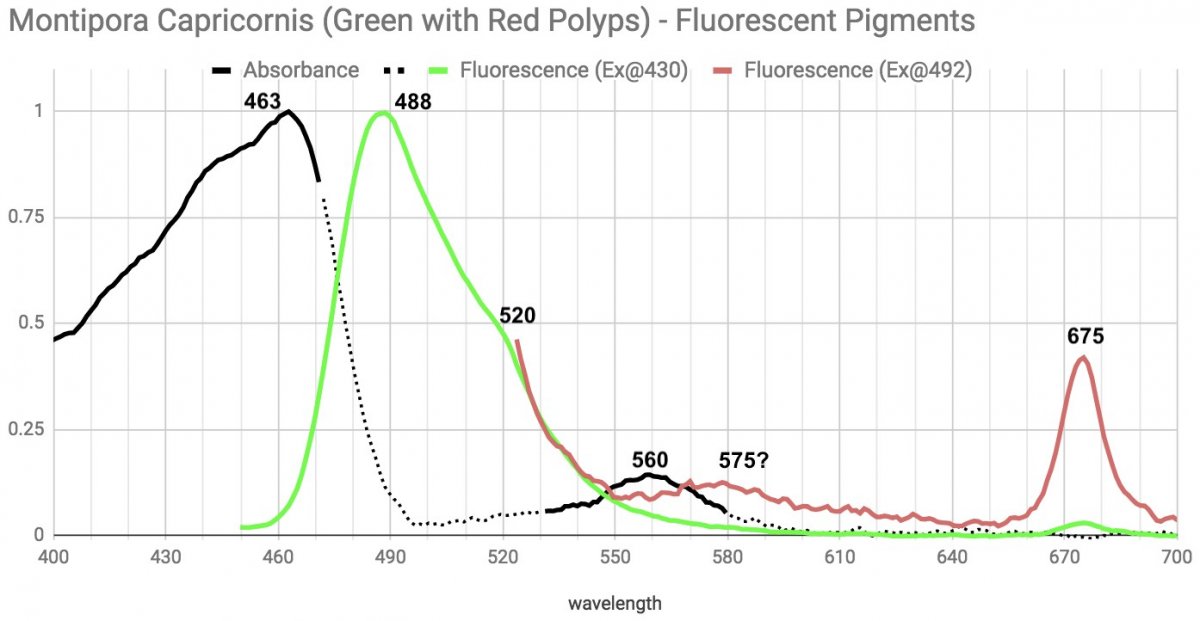
The green pigment here absorbs well from the extreme violet/UV up to the mid 400s, and emits green with the peak and shoulder feature again. There is a second absorbance that peaks around 560 but absorbs in the low 500s too that appears connected to the red fluorescence in the polyps. There's a possible orange emission around 575 (which is the dominant emission in the orange monti cap) and a strong emission at 675 where Chlorophyll fluoresces. See here for Dana's discussion(article) of a pocillopora damicornis emitting(pic) red in similar way.
Montipora Digitata.
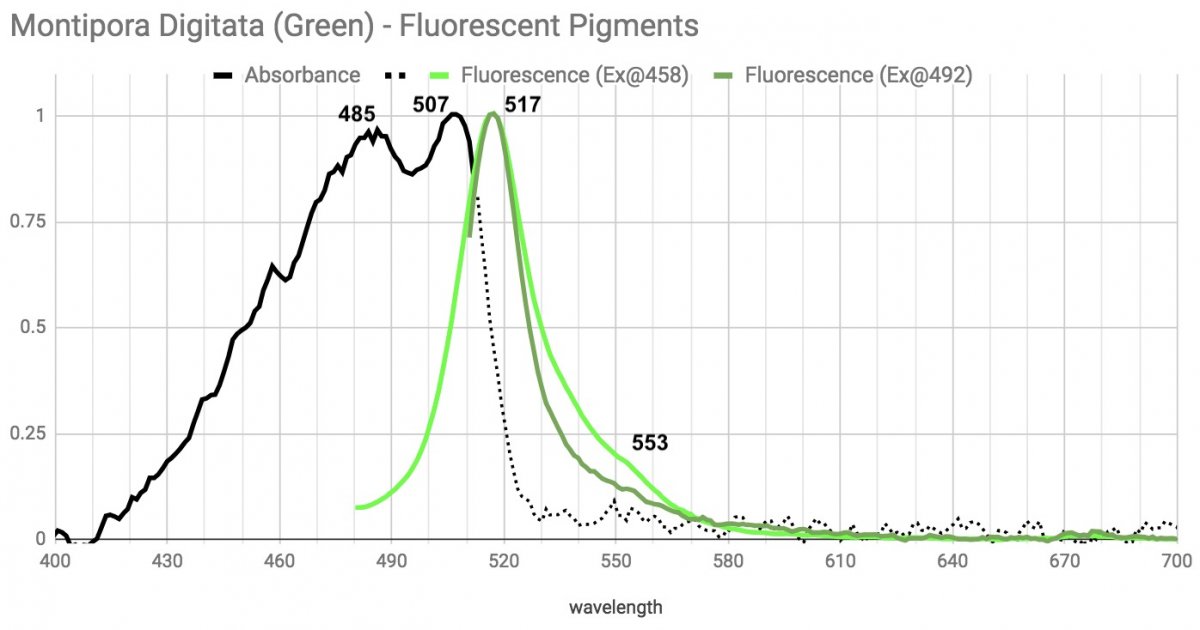
This solid green monti digi has a double peak or more likely, is two pigments. Excitation light at 458nm (and everything shorter wavelength than that) excites the familiar peak and shoulder emission pattern. But excitation at 492 - basically on the left absorbance peak, excites the main emission at 517 narrowly and strongly, but the shoulder at 553 disappears. This is another green pigment(s) that is almost totally unresponsive to extreme violet/UV.
Green Star Polyp.
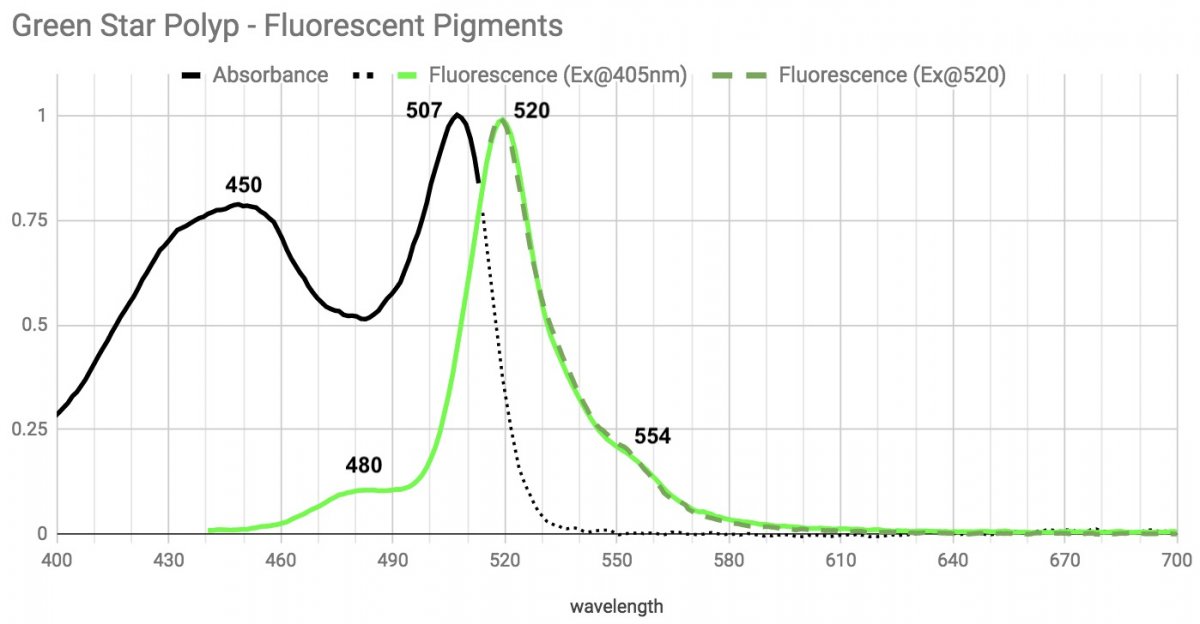
The weediest coral has the weirdest green fluorescent complex that I saw.
Possibly something like that may be happening here? The main emission peak (520) and shoulder (554) can be excited by light at the left absorbance (450) or the right absorption (507). But the left absorbance peak also generates light with a peak around 480, and that in turn would also excite the right absorbance peak.
(Through this, I've been thinking about what actually makes corals "pop," and how to quantify and evaluate the best light to accentuate particular coral pigments. But that discussion is for another post.)
Quite an impressive study. We might need a forum covering just the “science of the reef aquarium”.
Interesting thought about the light emission of one protein being absorb by another and causing a secondary emission. Is there something like an efficiency factor that would be make or break this idea? I assume not every photon would find its way to the right place. Can it actually happen? Would we actually be able to observe it? I have flowers in my garden with stunning colors. I wonder to what extent fluorescence is involved?
I anxiously await the next episode in this story.
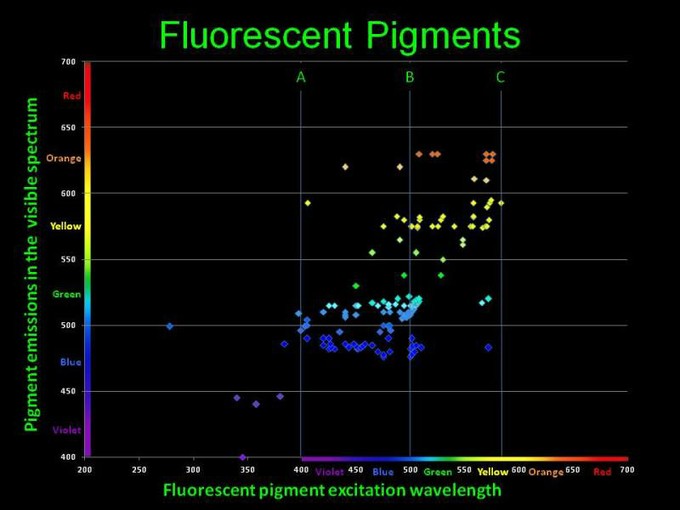
This irritates me a little. Dan Kelly produced this chart based on data that took me years to collect. It was then published in Advanced Aquarist in an article that I peer-reviewed. I was really interested in this and got in touch with Dan about his graph... Oh never mind. LOL.
- Joined
- May 22, 2016
- Messages
- 6,575
- Reaction score
- 10,162
What's up with the handful of points on the wrong side of the excitation - emission diagonal line?This irritates me a little. Dan Kelly produced this chart based on data that took me years to collect. It was then published in Advanced Aquarist in an article that I peer-reviewed. I was really interested in this and got in touch with Dan about his graph... Oh never mind. LOL.
There's a few points plotted there that have shorter wavelength emission peaks then the light they absorb. Is that accurate? Are they like catching two photons and emitting one?
Similar threads
- Replies
- 3
- Views
- 453


















