Is alkalinity just buffering capacity or am I missing something?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Alkalinity = buffering capacity?
- Thread starter Courtney Aldrich
- Start date
- Tagged users None
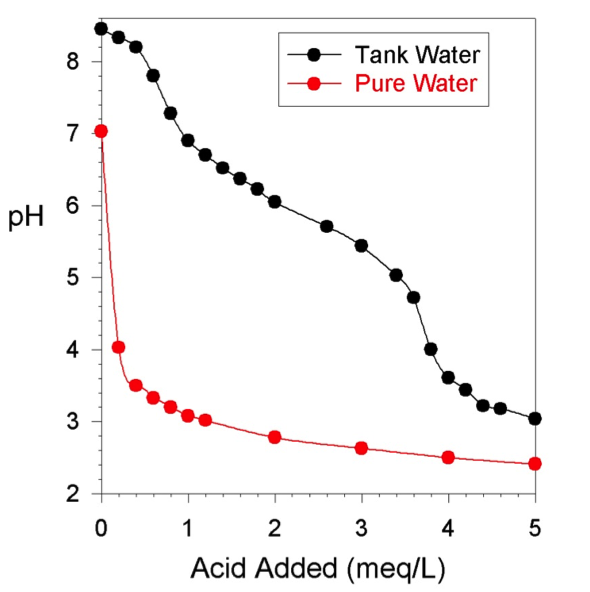
Typically we measure it in order to gauge how much bicarbonate is available to corals for their skeletal growth.
Tests generally measure total alkalinity however, which is basically the waters ability to resist become more acid. Bicarbonate which we care about measuring is the larger part of what buffers the water.
 www.reef2reef.com
www.reef2reef.com
Tests generally measure total alkalinity however, which is basically the waters ability to resist become more acid. Bicarbonate which we care about measuring is the larger part of what buffers the water.
What is Alkalinity?
Most reefkeepers know they need to measure alkalinity, and most know it has something to do with carbonate. But what is alkalinity exactly? Why is it important? How is it measured? This article will answer those questions and give you all of the...
 www.reef2reef.com
www.reef2reef.com
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,136
- Reaction score
- 63,473
As noted above, we measure it because bicarbonate or carbonate are harder to measure directly. We care about it mostly because it depletes fast and corals need it.
Alkalinity directly impacts buffering, but buffering is not a simple gauge of alkalinity. I discuss the actual math of buffering and alkalinity here:
 reefs.com
reefs.com
Alkalinity directly impacts buffering, but buffering is not a simple gauge of alkalinity. I discuss the actual math of buffering and alkalinity here:

Chemistry And The Aquarium: Boron In A Reef Tank
This article also discusses the sources and sinks for boron in reef tanks, and shows that while typical tanks may be slightly depleted in boron with respect to natural levels, most are not so significantly depleted that any correction is required. Nevertheless, how to test for and supplement...
Thank you for sharing this beautiful review on buffering capacity and boron. I'm amazed that you have written comprehensive articles on virtually every single reef chemistry topic, which are accessible and informative for beginners and PhD chemists alike. I would love to see you put everything together in a book. Perhaps a future project!
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,136
- Reaction score
- 63,473
Thanks!
One of the reasons I wrote many of the articles at a deeper chemistry level than most reefers care about is to try to move hard and fast chemistry principles out of the realm of "he said she said' sorts of arguments.
To some extent, that was the state of reef hobby chemistry when I became interested in the early 1990's. Even some of the big names in the hobby were making claims that were simply incorrect. By putting all the principles into an article on, say, "alkalinity" makes it a lot easier to build the case to ignore the folks claiming "X".
There was, and still is, a huge amount of reef lore that says "it worked for me, so try it" when in reality if one understood why it might have worked (or just be coincidence) in one tank, then one can better understand why it might or might not work in another.
Tap water is a perfect example. It is obvious to a chemist that not all tap water is alike, and a quick reading of the water supply reports for a variety of locations shows enough copper to be a problem in some cases. But it is not a problem in many others. So when folks say "tap water works fine" because "I use it and here's a picture of my reef tank", it takes more evidence to convince new reefers that there is a risk, and actual numbers from reports clarifies the risk. One of the complexities is that some folks think their tap water is really pure because it comes from (super pure springs, etc.) when in reality, copper often comes from your own pipes, so even your neighbor may have good water and you do not. The reef chemistry world is a constant battle against such misunderstandings. lol
One of the reasons I wrote many of the articles at a deeper chemistry level than most reefers care about is to try to move hard and fast chemistry principles out of the realm of "he said she said' sorts of arguments.
To some extent, that was the state of reef hobby chemistry when I became interested in the early 1990's. Even some of the big names in the hobby were making claims that were simply incorrect. By putting all the principles into an article on, say, "alkalinity" makes it a lot easier to build the case to ignore the folks claiming "X".
There was, and still is, a huge amount of reef lore that says "it worked for me, so try it" when in reality if one understood why it might have worked (or just be coincidence) in one tank, then one can better understand why it might or might not work in another.
Tap water is a perfect example. It is obvious to a chemist that not all tap water is alike, and a quick reading of the water supply reports for a variety of locations shows enough copper to be a problem in some cases. But it is not a problem in many others. So when folks say "tap water works fine" because "I use it and here's a picture of my reef tank", it takes more evidence to convince new reefers that there is a risk, and actual numbers from reports clarifies the risk. One of the complexities is that some folks think their tap water is really pure because it comes from (super pure springs, etc.) when in reality, copper often comes from your own pipes, so even your neighbor may have good water and you do not. The reef chemistry world is a constant battle against such misunderstandings. lol
Similar threads
- Replies
- 6
- Views
- 145
- Replies
- 15
- Views
- 352
- Replies
- 6
- Views
- 423
- Replies
- 43
- Views
- 840
New Posts
-
-
just a clown fish? They 8 cycling. Temp low?
- Latest: BigMonkeyBrain
-
-
-













