Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,529
- Reaction score
- 63,977
People always tell me i'm crazy for running my tanks like this, and admittedly, i only run some of them this way, but, i've had more growth with this method than almost anything else. They say don't chase ph?? naw, chase ph, with kalk...why not. PH shutoff which doses kalk or RODI depending. The only real black magic is figuring out what the PH shutoff needs to be to get to a target alk/cal range, but, with appropriate timeouts on my doser, i've made this quite safe.
Oddly enough, i'm wondering if your table might have answered the "black magic" bit some more
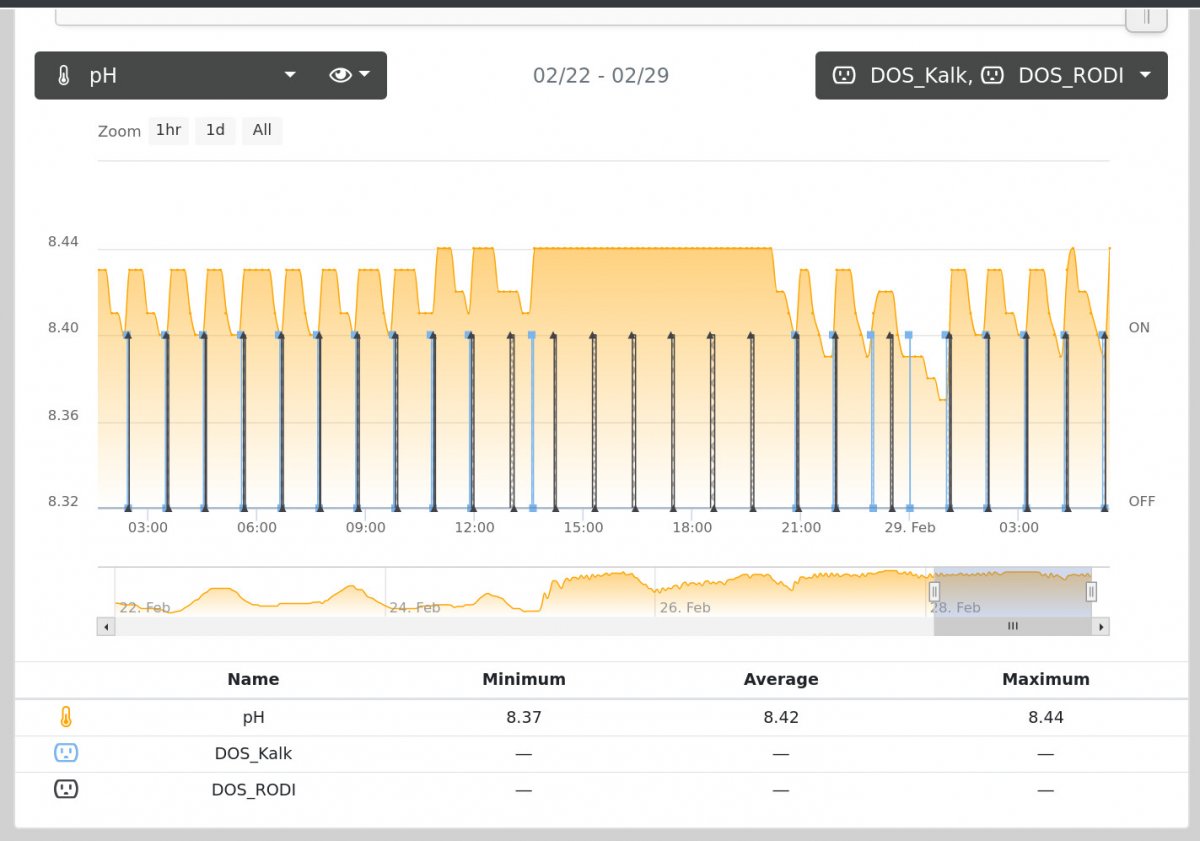
and here's the tank in question.
How stable is the alkalinity doing this?



















