sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
As part of a experiment on the effects of decomposition of organics I’ve added a defrost shrimp (5g) to a vessel with 100ml of tank water containing a very small amount of carbohydrates.
The goal of the experiment was to analyse the ammonia rise from the shrimp and the slow decomposition process due to low o2 in a small volume of water although it seems that the carbohydrates affected the end result in a way that I can’t understand, the ammonia rise never happened and instead ammonia was instantly converted to nitrite (1ppm plus) and nitrite converted to nitrates (220 plus)
question:
how did this happened, organic Carbon can only be useful during the denitrification process for denitrifying bacteria to my knowledge, nitrifying bacteria can’t use organic carbon in order to accelerate the nitrifying cycle and heterotrophic nitrifying bacteria is poorly studied for me to make any conclusion.
I have repeated the test with just tap water and the shrimp and the result at 8 hours was just the expected small amount of ammonia, it seems that the carbohydrates were the culprit.
Some more info on the experiment
experiment 1
Decomposition of a shrimp in a small vessel
• Duration 18 hours
• 5 grams defrosted cooked shrimp
• 100 ml tank water collected just after dosing reef actif
• ammonia 0 pppm
• nitrite 0
• nitrates 15ppm
• ph 8.1
Results:
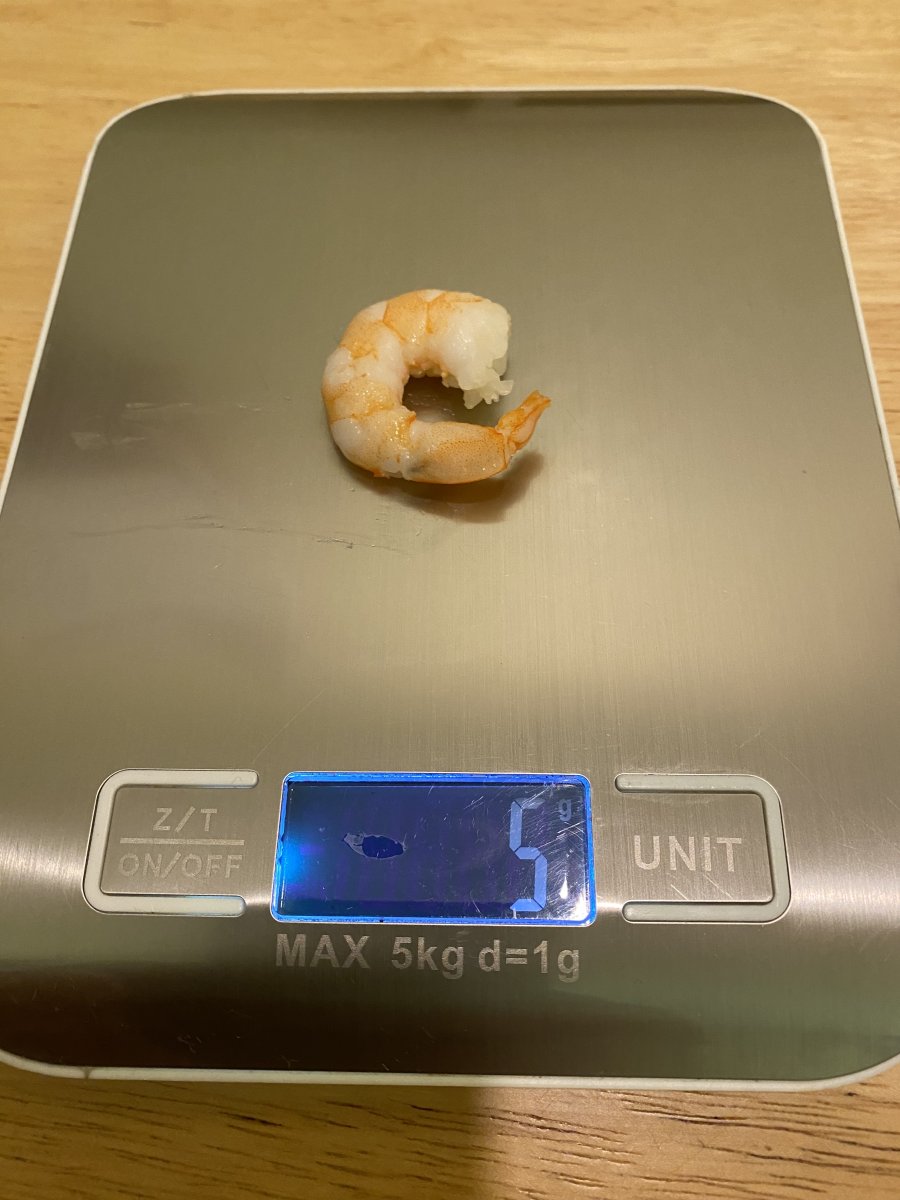
• Shrimp weigh 5 grams
• ammonia 0 ppm
• nitrite above 1 ppm
• nitrates 220 ppm plus (system runs at 15ppm
• ph 6
comments:
There was no weight loss, visually the shrimp still looked fresh, there was a cloudiness of the water in the vessel although ammonia was 0 ppm.
I was hoping to have an ammonia reading to illustrate this part of the test that would show the results of slow decomposition is ammonia. It seems that somehow the carbon source transformed all the ammonia to nitrite and nitrite to nitrates
will tag @MnFish1 and @Lasse to hear they’re views although if anyone knows this phenomenon or what I may have missed out please chime in.
pictures from experiment 1
0 hours beginning of experiment

18 hours end of experiment

18 hours end of experiment
18 hours ammonia 0 ppm

18 hours nitrite 1ppm plus

nitrates at 18 hours 220 plus (system nitrate 15ppm)

The goal of the experiment was to analyse the ammonia rise from the shrimp and the slow decomposition process due to low o2 in a small volume of water although it seems that the carbohydrates affected the end result in a way that I can’t understand, the ammonia rise never happened and instead ammonia was instantly converted to nitrite (1ppm plus) and nitrite converted to nitrates (220 plus)
question:
how did this happened, organic Carbon can only be useful during the denitrification process for denitrifying bacteria to my knowledge, nitrifying bacteria can’t use organic carbon in order to accelerate the nitrifying cycle and heterotrophic nitrifying bacteria is poorly studied for me to make any conclusion.
I have repeated the test with just tap water and the shrimp and the result at 8 hours was just the expected small amount of ammonia, it seems that the carbohydrates were the culprit.
Some more info on the experiment
experiment 1
Decomposition of a shrimp in a small vessel
• Duration 18 hours
• 5 grams defrosted cooked shrimp
• 100 ml tank water collected just after dosing reef actif
• ammonia 0 pppm
• nitrite 0
• nitrates 15ppm
• ph 8.1
Results:
• Shrimp weigh 5 grams
• ammonia 0 ppm
• nitrite above 1 ppm
• nitrates 220 ppm plus (system runs at 15ppm
• ph 6
comments:
There was no weight loss, visually the shrimp still looked fresh, there was a cloudiness of the water in the vessel although ammonia was 0 ppm.
I was hoping to have an ammonia reading to illustrate this part of the test that would show the results of slow decomposition is ammonia. It seems that somehow the carbon source transformed all the ammonia to nitrite and nitrite to nitrates
will tag @MnFish1 and @Lasse to hear they’re views although if anyone knows this phenomenon or what I may have missed out please chime in.
pictures from experiment 1
0 hours beginning of experiment
18 hours end of experiment
18 hours end of experiment
18 hours ammonia 0 ppm
18 hours nitrite 1ppm plus
nitrates at 18 hours 220 plus (system nitrate 15ppm)
Last edited:



















