- Joined
- Sep 21, 2018
- Messages
- 6,676
- Reaction score
- 7,171
A year ago, I became interested in carbon dosing because of the good things I heard about it, and the dire warnings (diminished capability to remove nitrate after dosing stops, encouragement of cyanobacteria and other nuisance organism growth, diminished biodiversity of the bio filter and proliferation of harmful bacteria). I took the plunge and started dosing my fish only system. The nitrate level was generally below 1 ppm and phosphate below 0.1 ppm. There was no nitrate issue. I dosed calcium acetate made from vinegar mixed with just enough solid kalkwasser to bring the pH to 10-11. I spent the year slowly increasing the daily dose, observing the system for a week to a month after each step increase. For this post I report data up to the not very large dose of 0.25 mL per gallon. I eventually achieved 2 mL/gallon, but that data is for another post. During this time, the only detected change was alkalinity consumption. Because the system contains only fish, alkalinity consumption should reflect only the metabolic activity of bacteria and algae, the system’s bio filter.
Alkalinity consumption was determined by measuring the alkalinity about once a week and dividing the difference by the number of elapsed days. I raised the alkalinity with a solution of sodium bicarbonate whenever it fell below 3 meq/L.
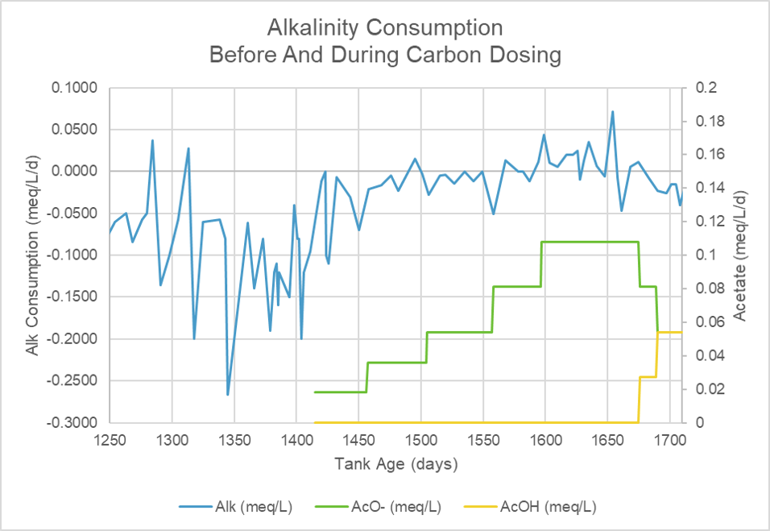
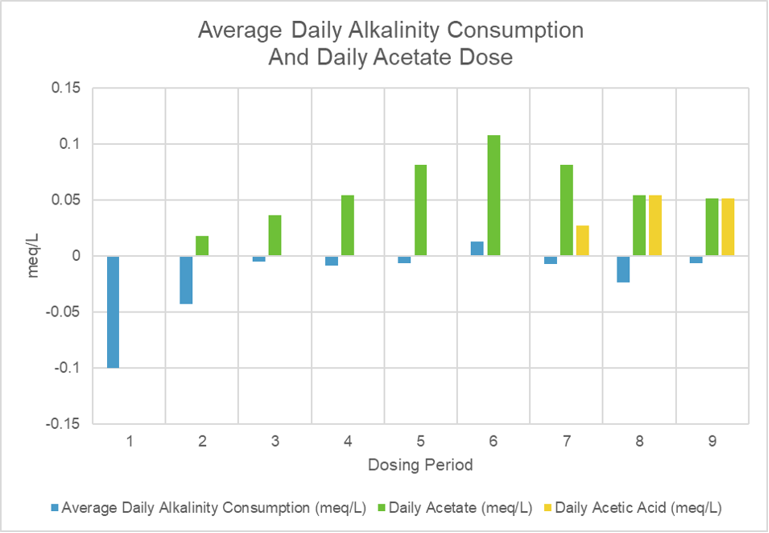
The first graph shows a highly variable alkalinity consumption rate before dosing and a much more consistent consumption rate after the initial small dose of acetate. The green line charts the size of the daily acetate dose while the yellow line charts the addition of acetic acid as I transitioned from dosing acetate to dosing straight acetic acid. The bar chart shows the average alkalinity consumption rate for each period between dose increases. The first period is the 164 days before dosing started. The reduction in the initial alkalinity consumption rate occurs with the first small dose. The reduction in consumption is larger than can be explained by the added alkalinity of the daily acetate dose. Eventually, the alkalinity of the acetate dose did seem to raise the system’s alkalinity (positive alkalinity consumption rate). This idea is supported by the observation of the return to a negative consumption rate during the transition from acetate to acetic acid dosing.
This dosing experiment has left me with a list of questions and a long reading list. Some of my questions:
Was the decrease in variability of alkalinity consumption rate a coincidence?
Is alkalinity consumption rate effected by other carbon sources, such as ethanol?
Does this alkalinity consumption effect occur in all marine aquaria?
Will a highly variable alkalinity consumption rate return when dosing is stopped?
Might alkalinity consumption rate be a useful diagnostic for the health of an aquarium bio filter?
With regard to this last question, the average alkalinity consumption rate of 0.1 meq/L/Day corresponds to a 10-20 g daily consumption of biomass based on ammonia nitrogen consumption. I feed the fish about that everyday. Maybe just a coincidence. Here is the reference I used for the calculation
https://www.researchgate.net/public...al_of_ammonia-nitrogen_in_aquaculture_systems
Your thoughts and comments would be welcome.
Dan
Alkalinity consumption was determined by measuring the alkalinity about once a week and dividing the difference by the number of elapsed days. I raised the alkalinity with a solution of sodium bicarbonate whenever it fell below 3 meq/L.
The first graph shows a highly variable alkalinity consumption rate before dosing and a much more consistent consumption rate after the initial small dose of acetate. The green line charts the size of the daily acetate dose while the yellow line charts the addition of acetic acid as I transitioned from dosing acetate to dosing straight acetic acid. The bar chart shows the average alkalinity consumption rate for each period between dose increases. The first period is the 164 days before dosing started. The reduction in the initial alkalinity consumption rate occurs with the first small dose. The reduction in consumption is larger than can be explained by the added alkalinity of the daily acetate dose. Eventually, the alkalinity of the acetate dose did seem to raise the system’s alkalinity (positive alkalinity consumption rate). This idea is supported by the observation of the return to a negative consumption rate during the transition from acetate to acetic acid dosing.
This dosing experiment has left me with a list of questions and a long reading list. Some of my questions:
Was the decrease in variability of alkalinity consumption rate a coincidence?
Is alkalinity consumption rate effected by other carbon sources, such as ethanol?
Does this alkalinity consumption effect occur in all marine aquaria?
Will a highly variable alkalinity consumption rate return when dosing is stopped?
Might alkalinity consumption rate be a useful diagnostic for the health of an aquarium bio filter?
With regard to this last question, the average alkalinity consumption rate of 0.1 meq/L/Day corresponds to a 10-20 g daily consumption of biomass based on ammonia nitrogen consumption. I feed the fish about that everyday. Maybe just a coincidence. Here is the reference I used for the calculation
https://www.researchgate.net/public...al_of_ammonia-nitrogen_in_aquaculture_systems
Your thoughts and comments would be welcome.
Dan
















