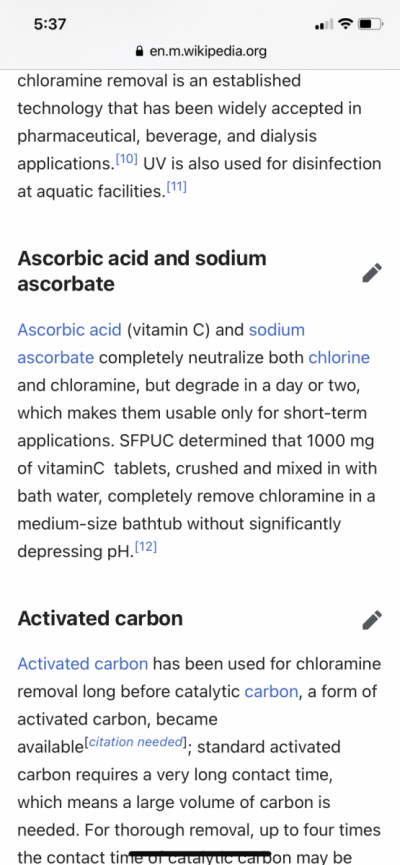
From my understanding activated carbon in a RO unit does not remove all of the chloramines in the tap water.
Is there any benefit of using a dedicated dechlorinator Eg Prime vs Ascorbic Acid?
Thanks
Is there any benefit of using a dedicated dechlorinator Eg Prime vs Ascorbic Acid?
Thanks