Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dinoflagellates - dinos a possible cure!? Follow along and see!
- Thread starter twilliard
- Start date
- Tagged users None
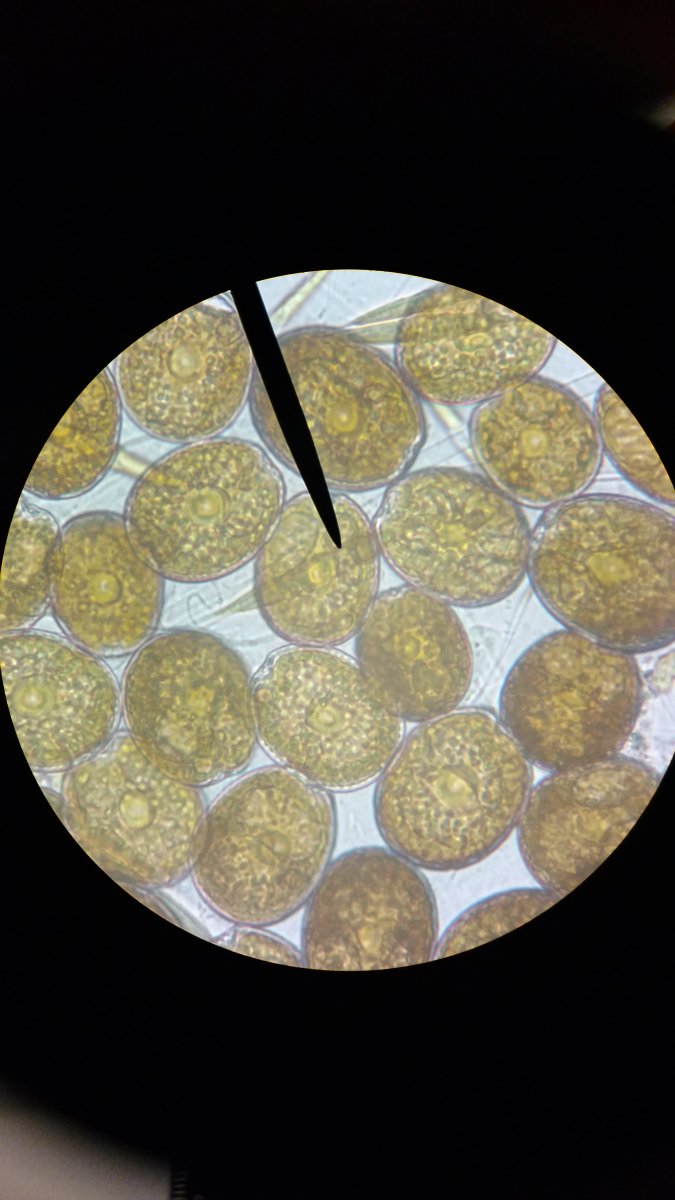
Wow, @Salty Pickle, you have a nice scope! Really clear pics (unlike mine!) At what magnification were your images taken? From where in the tank did the samples come? What sort of movement do they make? Gliding or something else? LOOKS to me like a prorocentrum (lacking the tongue-like groove of an amphidinium)...but I could be wrong!Could somebody please help me ID so I can start treatment. Thank you for your time.

You can also look here: http://www.algaeid.com/identification/ or here: http://species-identification.org/index.php?selectie=24&hoofdgroepen_pad=,3,24&groep=Dinoflagellates&eerst_getoonde_soort=0 to see if that helps with ID.
- Joined
- May 22, 2016
- Messages
- 6,570
- Reaction score
- 10,150
Coolia I'd describe as benthic, in my tank they first formed in the sand, then later could be found on rocks with bubbles and short strings. Ostreopsis I've seen described as benthic (surface) or epiphytic (attached to other living matter: algae & coral). If any of our dinos were truly planktonic, then our skimmers would keep them from ever achieving noticeable numbers.We don't have many in our tank, never more than a couple in my field of view in the scope. Are coolia benethic like amphidinium, or planktonic like ostreopsis?
That really matches my personal experience. I have a jawfish, sea cucumber, a conch and a fleet of ceriths (and many pods) that have been with me through almost the whole Dino ordeal and are fine. I never let my ostreopsis get significant numbers, and amphidinium and Coolia are much lower toxicity.We had no deaths (not even snails or hermits) in our tank from the ostreopsis infestation (though we kicked it before it was too bad), so our variety must be lower toxicity than some. If coolia is similar in toxicity as you mentioned, that may mean I should rethink the livestock order I was going to place tomorrow. We were going to get a couple tiger conchs and a diamond watchman goby for sand aeration. Any ideas if low quantities of coolia ingestion would be harmful to these guys? Any sand sifters/cleaners in your tank when coolia was present?
Thanks very much!
Here's a cool paper that's new to me. It's about Coolia and ostreopsis showing up in New Zealand. Good pics, and toxicity experiments showing Coolia toxin not even comparable to ostreopsis.
http://www.tandfonline.com/doi/pdf/10.1080/00288330.2000.9516939
One thing I feel like I need to add here at the risk of getting way too long. When talking about predation/grazing on slightly toxic dinos - there is a difference between "eats dinos" and "can grow on a diet of dinos"
Tons of things will eat dinos, but research has not found anything that can grow on a diet of dinos. If you have grazers that will consume dinos, you must provide other food for them or they will die. Dinos ability to crowd out other microlife complicates this.
My tank I ran high light (including AM sun), lots of macroalgae directly on sand bed, high nutrients (dirty), heavy feeding, and dumping skimmer cup back in tank (no export).
- Joined
- May 22, 2016
- Messages
- 6,570
- Reaction score
- 10,150
At what magnification were your images taken? From where in the tank did the samples come? What sort of movement do they make? Gliding or something else? LOOKS to me like a prorocentrum
Yep.
And here's a fun trick I picked up when trying to ballpark sizes in a scope. Look at the diatoms. The small ones that have a pigmented center that tapers to a colorless thin thread on either end - the pigmented central part of those is almost always between 20-30 microns long. They are some kind of pinnate diatom, but I forget more specific name. Find a few of those, and an average size one is a 25 micron scale-bar!
Edit:

Last edited:
Very cool tip with the diatoms, @taricha! I had seen them in the background of his images, but didn't think to use them for size reference; hence my question about his magnification. It's always fun to learn new things; thanks!Yep.
And here's a fun trick I picked up when trying to ballpark sizes in a scope. Look at the diatoms. The small ones that have a pigmented center that tapers to a colorless thin thread on either end - the pigmented central part of those is almost always between 20-30 microns long. They are some kind of pinnate diatom, but I forget more specific name. Find a few of those, and an average size one is a 25 micron scale-bar!
Edit:
Great input on your sand-dwelling/sifting critters and that they survived your various dino blooms. We have an established (3+ years) 125 gallon reef tank with 1 1/2" sand bed (no refugium in this set up due to space restrictions), and plenty of pods/microfauna in the sand to support the conch and diamond watchman...definitely were not getting them to eat dino cells, just to aerate the sand and help keep it more clean of detritus. I do appreciate your caution, as we never want to buy creatures doomed for starvation in our tank! Even though we don't see blooms of dinos in our tank anymore, they are there in small numbers under the scope, and if some of them get eaten, that's all the better!Coolia I'd describe as benthic, in my tank they first formed in the sand, then later could be found on rocks with bubbles and short strings. Ostreopsis I've seen described as benthic (surface) or epiphytic (attached to other living matter: algae & coral). If any of our dinos were truly planktonic, then our skimmers would keep them from ever achieving noticeable numbers.
That really matches my personal experience. I have a jawfish, sea cucumber, a conch and a fleet of ceriths (and many pods) that have been with me through almost the whole Dino ordeal and are fine. I never let my ostreopsis get significant numbers, and amphidinium and Coolia are much lower toxicity.
Here's a cool paper that's new to me. It's about Coolia and ostreopsis showing up in New Zealand. Good pics, and toxicity experiments showing Coolia toxin not even comparable to ostreopsis.
http://www.tandfonline.com/doi/pdf/10.1080/00288330.2000.9516939
One thing I feel like I need to add here at the risk of getting way too long. When talking about predation/grazing on slightly toxic dinos - there is a difference between "eats dinos" and "can grow on a diet of dinos"
Tons of things will eat dinos, but research has not found anything that can grow on a diet of dinos. If you have grazers that will consume dinos, you must provide other food for them or they will die. Dinos ability to crowd out other microlife complicates this.
My tank I ran high light (including AM sun), lots of macroalgae directly on sand bed, high nutrients (dirty), heavy feeding, and dumping skimmer cup back in tank (no export).
I will be interested in reading the abstract; thanks for including it for me.
Wow, @Salty Pickle, you have a nice scope! Really clear pics (unlike mine!) At what magnification were your images taken? From where in the tank did the samples come? What sort of movement do they make? Gliding or something else? LOOKS to me like a prorocentrum (lacking the tongue-like groove of an amphidinium)...but I could be wrong!Others may have a more accurate opinion, as I'm new at this.
You can also look here: http://www.algaeid.com/identification/ or here: http://species-identification.org/index.php?selectie=24&hoofdgroepen_pad=,3,24&groep=Dinoflagellates&eerst_getoonde_soort=0 to see if that helps with ID.
Sorry for the lack of info, I was in a rush.
I am using the LEICA CME. The first pic objective was 40x/0.65 and the second objective was 10x/0.25. The eye piece is 10x/18. they don't make very much movement, glide would be the best description. I couldn't tell if it was heat from the illuminator or water movement between the slide and slide cover. I collected 5 samples from a 125 gallon tank, all 4 corners and center, same organism. I am making an assumption they are dinos because it has formed a thick mat over the entire sand bed, brown in color, strands with bubbles forming at the tips of the strands. I attempted a water change and siphoned all the gravel so that the sand was white again. By the time I got to the other side of the tank patches of brown began to form again, which I thought were spots I missed, but then started to form on the other side that I knew I had just siphoned. By morning the entire sanded was covered again. Everyday it gets progressively worse, and my snails are dying (3 nassarius, 4 trochus) and corals are irritated by the strands. I tried a 3 day lights out which had virtually no effect. I have been running BRS HD GFO and ROX 0.8 carbon, chaetomorpha in the refugium, took the biopellets offline, cut down lighting to 4 hours, cut feeding down from 1-2x a day to every other day (anthias are not happy at all), increased skimming to be very wet, changed to 225 micron Red Sea filter socks (which clogs within 1-2 days), and adding Vibrant. Due to my job situation I can only siphon the crud off the gravel every 3-4 days which also equates to about 30 gallon water changes every 3-4 days. After almost 3 weeks there is very little change. Fish are starving and corals are really upset. I did a head count and all fish are accounted for so there is nothing decaying behind the rocks anywhere. I blasted the rocks to get any garbage that has collected in the cracks and set my MP40's to NTM. Clearly I am missing something and like an idiot I started doing a whole bunch of treatments without identifying what I was fighting. I got sucked into a panic and it directed my behavior. To make matters worse I am out of test kits, I kept telling myself that I would have this under control before I ran out, well that didn't happen either. my last test (Red Sea Kits) was March 13th and my parameters were:
NO3 = 3ppm
PO4 = 0.08ppm
CA = 450
MG = 1340
KH = 8.5
PH= 8.23
TEMP = 77.9
I am hoping with a proper identification I can treat intelligently and not emotionally. I would like to thank the community for always being so awesome and willing to help. Happy reefing!
Last edited:
heres what they look like in the tank. I was having issues collecting samples that didn't pull sand with it and crack the slide covers so I used surgical scissors to snip the threads and allowed the bubble to bring it to the surface and a pipette to put it on the slide. Tada..no sand, lol.

Last edited:
@Salty Pickle, you must be so frustrated with the havoc dinos are having in your tank at the moment. I am so sorry, and I know how helpless it feels to battle these buggers. Sometimes it seems as if nothing is going right. It's not that you're missing something; it's that dinos are so, so hard to treat and eradicate! It's simply trial and error, unfortunately, to see what works and doesn't work in your tank. There is not a "do this if you have this type of dino, but do this if you have another type." Sadly. As you can see in this thread, people try many things, and they may or may not work for a specific tank. There are clearly other variables in play that reefers don't know how to manipulate to their advantage yet..Sorry for the lack of info, I was in a rush.
I am using the LEICA CME. The first pic objective was 40x/0.65 and the second objective was 10x/0.25. The eye piece is 10x/18. they don't make very much movement, glide would be the best description. I couldn't tell if it was heat from the illuminator or water movement between the slide and slide cover. I collected 5 samples from a 125 gallon tank, all 4 corners and center, same organism. I am making an assumption they are dinos because it has formed a thick mat over the entire sand bed, brown in color, strands with bubbles forming at the tips of the strands. I attempted a water change and siphoned all the gravel so that the sand was white again. By the time I got to the other side of the tank patches of brown began to form again, which I thought were spots I missed, but then started to form on the other side that I knew I had just siphoned. By morning the entire sanded was covered again. Everyday it gets progressively worse, and my snails are dying (3 nassarius, 4 trochus) and corals are irritated by the strands. I tried a 3 day lights out which had virtually no effect. I have been running BRS HD GFO and ROX 0.8 carbon, chaetomorpha in the refugium, took the biopellets offline, cut down lighting to 4 hours, cut feeding down from 1-2x a day to every other day (anthias are not happy at all), increased skimming to be very wet, changed to 225 micron Red Sea filter socks (which clogs within 1-2 days), and adding Vibrant. Due to my job situation I can only siphon the crud off the gravel every 3-4 days which also equates to about 30 gallon water changes every 3-4 days. After almost 3 weeks there is very little change. Fish are starving and corals are really upset. I did a head count and all fish are accounted for so there is nothing decaying behind the rocks anywhere. I blasted the rocks to get any garbage that has collected in the cracks and set my MP40's to NTM. Clearly I am missing something and like an idiot I started doing a whole bunch of treatments without identifying what I was fighting. I got sucked into a panic and it directed my behavior. To make matters worse I am out of test kits, I kept telling myself that I would have this under control before I ran out, well that didn't happen either. my last test (Red Sea Kits) was March 13th and my parameters were:
NO3 = 3ppm
PO4 = 0.08ppm
CA = 450
MG = 1340
KH = 8.5
PH= 8.23
TEMP = 77.9
I am hoping with a proper identification I can treat intelligently and not emotionally. I would like to thank the community for always being so awesome and willing to help. Happy reefing!
In my post this morning, and echoed by @taricha above, it looks from your scope pics that you have a dino of the prorocentrum species in your tank. (I had asked about magnification because I wasn't sure of my ID, and sometimes size of the cell helps. Again, really nice scope!) Looking back at the links I posted for you to check out about prorocentrum, it looks like they are toxic (you see that with CUC deaths), benthic (occur more at the bottom of the water column) and are armored. This means that adding a UV to your system is unlikely to help your type of dino. Looking at the other link I gave you, the scientist said he had not seen a prorocentrum bloom in an aquarist's tank, though he has found them in tanks. Looks like you're unluckily unusual in they type of dino bloom you have, and not as many people to look to for advice on how to treat. Sorry to be the bearer of bad news in that respect.
But, DON'T give up. You've been dealing with this for about 3 weeks, right? Ideas to try:
Others have used the "dirty" method on dinos: heavy feeding, no skimming (or even skimming and dumping skim mate back into the tank), adding live phytoplankton and copepods (folks like Algae Barn) to feed microscopic critters in the tank that can maybe outcompete/consume dinos. Raise nutrient levels (NO3 and PO4, for instance) by dosing; adding bacteria in a bottle from a variety of manufacturers to add new bacteria populations to the tank.
Dosing peroxide, metro, bleach (remove fish, etc first)...I'm not knowledgeable in those last two; you'll have to read this thread or ask others to chime in who have used these)
Continue dosing Vibrant (what is your dosing schedule now?)
This is a lot for now; ask questions and you'll get lots of folks to help out as they have knowledge! Good luck!
Good idea for removal...never thought about allowing the bubble to bring it to the surface! We used long tongs/pipette, with varying success...depending on how well I'd get ahold of it with tweezers!heres what they look like in the tank. I was having issues collecting samples that didn't pull sand with it and crack the slide covers so I used surgical scissors to snip the threads and allowed the bubble to bring it to the surface and a pipette to put it on the slide. Tada..no sand, lol.

- Joined
- May 22, 2016
- Messages
- 6,570
- Reaction score
- 10,150
@Salty Pickle...it looks like they are toxic (you see that with CUC deaths), benthic (occur more at the bottom of the water column) and are armored. This means that adding a UV to your system is unlikely to help your type of dino. Looking at the other link I gave you, the scientist said he had not seen a prorocentrum bloom in an aquarist's tank, though he has found them in tanks. Looks like you're unluckily unusual in they type of dino bloom you have, and not as many people to look to for advice on how to treat.
Like you said, not much experience with a full prorocentrum bloom, so a lot of unanswered Qs.
UV: the only tank dino we know that doesn't go into the water column at night is the common amphidinium species (it goes down into sand slightly). Seems they all move somewhere in a daily vertical migration. It might be a safer assumption that these do leave the sand at night, and UV in-tank - for maximum contact is the first thing I'd try, in part to answer that Q. (+carbon & skimming to remove toxins in case it does work)
(Edit: I dunno the vibrant/UV interaction, but since it has a living component, I'm guessing UV would at least partially disable vibrant.)
@Salty Pickle How does the sand look right at lights-on? Guessing it's much clearer.
Thanks for the tank shots. Good to see what a big prorocentrum bloom looks like.
If you took sand samples, based on those pics, and your low nutrients, I'll be willing to say that Dino cells will massively outnumber every other form of visible life. If you introduce pods, snails, grazers etc with the sand in that state, grazers will die rapidly.
Like you said, not much experience with a full prorocentrum bloom, so a lot of unanswered Qs.
UV: the only tank dino we know that doesn't go into the water column at night is the common amphidinium species (it goes down into sand slightly). Seems they all move somewhere in a daily vertical migration. It might be a safer assumption that these do leave the sand at night, and UV in-tank - for maximum contact is the first thing I'd try, in part to answer that Q. (+carbon & skimming to remove toxins in case it does work)
(Edit: I dunno the vibrant/UV interaction, but since it has a living component, I'm guessing UV would at least partially disable vibrant.)
@Salty Pickle How does the sand look right at lights-on? Guessing it's much clearer.
Thanks for the tank shots. Good to see what a big prorocentrum bloom looks like.
If you took sand samples, based on those pics, and your low nutrients, I'll be willing to say that Dino cells will massively outnumber every other form of visible life. If you introduce pods, snails, grazers etc with the sand in that state, grazers will die rapidly.
@taricha, that is great news about the possibility of success with UV for @Salty Pickle! I knew amphibidium buried in the sand at night, and thought other more benthic ones would, too. Clearly I need more study before attempting to advice others!
For clarity, you don't think adding pods or phytoplankton at this point would be potentially helpful for @Salty Pickle. Clearly no additional CUC critters that would perish from the dinos. Do you think dosing NO3 to get nutrients up a bit will help or hurt? I know that means more for dinos to use, but also more for beneficial life to use, as well. I know there are different schools of thought on this (clean vs dirty methods), and I don't know which may be "better" in this case.
- Joined
- May 22, 2016
- Messages
- 6,570
- Reaction score
- 10,150
Not until dinos are reduced in number significantly, and a lot of other stuff is provided to eat.For clarity, you don't think adding pods or phytoplankton at this point would be potentially helpful for @Salty Pickle. Clearly no additional CUC critters that would perish from the dinos.
Some treatments are more in line with clean, some more with dirty. This is a lot of unprovable conjecture on my part.Do you think dosing NO3 to get nutrients up a bit will help or hurt? I know that means more for dinos to use, but also more for beneficial life to use, as well. I know there are different schools of thought on this (clean vs dirty methods), and I don't know which may be "better" in this case.
Chemical like vibrant, peroxide, bleach and UV, sand bed removal etc are closer to clean. Those nutrient levels say clean is where Salty's tank is now.
The methods of predation, grazing, and outcompeting, pods+phyto, adding macroalgae all in line with dirty method, and N + P should be elevated. (Although I'd do UV - at least at night - with dirty method too)
I like dirty. I dunno how to keep a clean tank in a dino-free stable state. Others can and do.
Salty's tank is a million miles from dirty right now. His nutrients are super low, his sand is a biodiversity desert. No grazers, and nothing for them to eat if he added them.
I thought I would post what seems to have worked for me. I was battling ostreopsis ovata. I did all the usual, vibrant, lights out, no water changes, peroxide, reduced lighting, etc. I could knock them back, but over time they would come back. I ended up doing another blackout, followed by once a week doses of 30% hydrogen peroxide, vs the 3% you get at the drug store. I then got some live rock and sand from different sources in an effort to boost biodiversity. With the exception of a few snails, all livestock tolerated the treatment, though I cannot say if this would be true for all. I am hesitant to say I have won the battle, but it has been weeks since dinos have reared their head. Maybe this approach will help someone, I wouldn't wish this scourge on anyone.
Makes sense!Not until dinos are reduced in number significantly, and a lot of other stuff is provided to eat.
Some treatments are more in line with clean, some more with dirty. This is a lot of unprovable conjecture on my part.
Chemical like vibrant, peroxide, bleach and UV, sand bed removal etc are closer to clean. Those nutrient levels say clean is where Salty's tank is now.
The methods of predation, grazing, and outcompeting, pods+phyto, adding macroalgae all in line with dirty method, and N + P should be elevated. (Although I'd do UV - at least at night - with dirty method too)
I like dirty. I dunno how to keep a clean tank in a dino-free stable state. Others can and do.
Salty's tank is a million miles from dirty right now. His nutrients are super low, his sand is a biodiversity desert. No grazers, and nothing for them to eat if he added them.
@sowellj, congrats on your success; thanks for sharing what worked for your tank...wouldn't it be nice to have a "dino prescription" we could just follow and know we'd be successful? Fingers crossed for you that yours will continue to remain at bay!I thought I would post what seems to have worked for me. I was battling ostreopsis ovata. I did all the usual, vibrant, lights out, no water changes, peroxide, reduced lighting, etc. I could knock them back, but over time they would come back. I ended up doing another blackout, followed by once a week doses of 30% hydrogen peroxide, vs the 3% you get at the drug store. I then got some live rock and sand from different sources in an effort to boost biodiversity. With the exception of a few snails, all livestock tolerated the treatment, though I cannot say if this would be true for all. I am hesitant to say I have won the battle, but it has been weeks since dinos have reared their head. Maybe this approach will help someone, I wouldn't wish this scourge on anyone.
So happy for you! How much of peroxide were you using? What kind of dinos?I thought I would post what seems to have worked for me. I was battling ostreopsis ovata. I did all the usual, vibrant, lights out, no water changes, peroxide, reduced lighting, etc. I could knock them back, but over time they would come back. I ended up doing another blackout, followed by once a week doses of 30% hydrogen peroxide, vs the 3% you get at the drug store. I then got some live rock and sand from different sources in an effort to boost biodiversity. With the exception of a few snails, all livestock tolerated the treatment, though I cannot say if this would be true for all. I am hesitant to say I have won the battle, but it has been weeks since dinos have reared their head. Maybe this approach will help someone, I wouldn't wish this scourge on anyone.
I was dosing 30% h2o2 at 1 ml per 10 gallon. I am a chemist so I have access to this grade of h202. In theory this would be the same as dosing regular h202 at 1 ml per gallon. Extreme, but when the alternative is a tank breakdown, about anything is worth a try. Dinos were O Ovata. I also only dosed 1x a week, with the idea that if they went dormant after the first dose I wouldn't be accomplishing anything with subsequent dosing and wanted to wait for them to remerge before I hit them again with the peroxide. I don't know if I have won, but it has been a while since I have seen dinosaur under a scope.So happy for you! How much of peroxide were you using? What kind of dinos?
Im dosing 2 ml por 10 gallons now and made blackout for 72 hours and today is the fist day I will see the results, hope everything is ok. I also have O. Ovata. You made the dosis with your livestock in it?I was dosing 30% h2o2 at 1 ml per 10 gallon. I am a chemist so I have access to this grade of h202. In theory this would be the same as dosing regular h202 at 1 ml per gallon. Extreme, but when the alternative is a tank breakdown, about anything is worth a try. Dinos were O Ovata. I also only dosed 1x a week, with the idea that if they went dormant after the first dose I wouldn't be accomplishing anything with subsequent dosing and wanted to wait for them to remerge before I hit them again with the peroxide. I don't know if I have won, but it has been a while since I have seen dinosaur under a scope.
Similar threads
- Replies
- 4
- Views
- 151
- Replies
- 9
- Views
- 554
- Replies
- 6
- Views
- 401
- Replies
- 2
- Views
- 385

















