we could chat about that in your other thread though vs here. if you're determined to continue you can, this was my offer to keep the thread here laser clean as it was recently. we could consider other theories there vs here, it was reasonable to suggest.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Does Prime actually "Detoxify" free ammonia, NH3?
- Thread starter taricha
- Start date
- Tagged users None
What I can say is that I cycled my very first QT with a clown and Prime. I didn't change the water until ammonia was around 2ppm. I just followed an article online. I understand though that a clown can take a lot. With that said, I have been playing it safe and doing water changes during my TTM and throwing in some Prime for the hell of it. I had to overfeed because my fishes weren't eating initially so had a little ammonia.
- Joined
- May 22, 2016
- Messages
- 6,542
- Reaction score
- 10,099
One thing that Prime gets wrongfully accused of is slowing a tank cycle.
Some say that since it claims to "bind" ammonia, then the ammonia processing to nitrite and nitrate is slowed by adding Prime. This is incorrect. Here is a demonstration.
I took some tank water, and dosed it to ~1ppm ammonia and split it into three 100mL samples. Two of the three samples got sand from a small cycled tank to process the ammonia.
bottle 1: Tank water + ammonia + Prime (no sand)
bottle 2: Tank water + ammonia (2% sand)
bottle 3: Tank water + ammonia + Prime (2% sand)
left Chart is ammonia testing with API total ammonia. Right chart is Nitrite. Green dashed lines are when Prime was added.
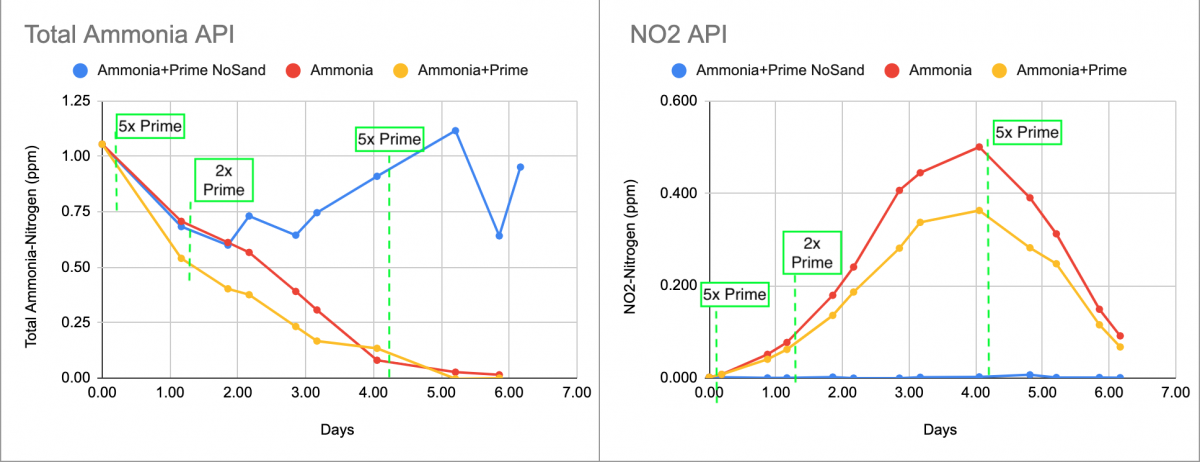
First the ammonia chart. It's quite messy, but it's clear that the samples with sand processed ammonia pretty similarly - perhaps exactly the same, but Prime makes that hard to see. The Prime clearly interfered with the total ammonia test somewhat, and the interference was erratic and unpredictable in the sample without sand. (Perhaps the sand samples broke down the Prime faster and decreased the interfering effect)
On to the Nitrite graph on the right. It's clean and requires almost no explanation. Nitrite starts being produced at the same time, with or without big doses of Prime, similarly it reaches peak NO2 value at the same time and the NO2 gets cleared at the same time - with or without Prime. Neither the bacteria oxidizing Ammonia->NO2 nor those oxidizing NO2->NO3 are slowed in the slightest by repeated big doses of Prime.
So attempts to blame (saltwater) cycling issues on Prime seem totally unfounded. It may make the tests (especially ammonia) harder to interpret - but it doesn't affect the ammonia nitrification process in the slightest. Such explanations from bottle bacteria sellers shouldn't be accepted.
Some say that since it claims to "bind" ammonia, then the ammonia processing to nitrite and nitrate is slowed by adding Prime. This is incorrect. Here is a demonstration.
I took some tank water, and dosed it to ~1ppm ammonia and split it into three 100mL samples. Two of the three samples got sand from a small cycled tank to process the ammonia.
bottle 1: Tank water + ammonia + Prime (no sand)
bottle 2: Tank water + ammonia (2% sand)
bottle 3: Tank water + ammonia + Prime (2% sand)
left Chart is ammonia testing with API total ammonia. Right chart is Nitrite. Green dashed lines are when Prime was added.
First the ammonia chart. It's quite messy, but it's clear that the samples with sand processed ammonia pretty similarly - perhaps exactly the same, but Prime makes that hard to see. The Prime clearly interfered with the total ammonia test somewhat, and the interference was erratic and unpredictable in the sample without sand. (Perhaps the sand samples broke down the Prime faster and decreased the interfering effect)
On to the Nitrite graph on the right. It's clean and requires almost no explanation. Nitrite starts being produced at the same time, with or without big doses of Prime, similarly it reaches peak NO2 value at the same time and the NO2 gets cleared at the same time - with or without Prime. Neither the bacteria oxidizing Ammonia->NO2 nor those oxidizing NO2->NO3 are slowed in the slightest by repeated big doses of Prime.
So attempts to blame (saltwater) cycling issues on Prime seem totally unfounded. It may make the tests (especially ammonia) harder to interpret - but it doesn't affect the ammonia nitrification process in the slightest. Such explanations from bottle bacteria sellers shouldn't be accepted.
@taricha Yes sure this test is at 1.1ppm and .5ppm +/- though. Now test this at levels phd doc says it inhibits growth. +5 ppm.
Hold on missing some nitrogen in these charts. Where did it go at day 4? Total nitrogen in day 1 .8892 mg/L N in NH3. Total nitrogen day 4 .2159 mg/L N in NH3+NO2 Do you have nitrate data on day 4? Where did the .6733 mg/L N go?
- Joined
- May 22, 2016
- Messages
- 6,542
- Reaction score
- 10,099
here's more info on nitrogen accounting....Hold on missing some nitrogen in these charts. Where did it go at day 4? Total nitrogen in day 1 .8892 mg/L N in NH3. Total nitrogen day 4 .2159 mg/L N in NH3+NO2 Do you have nitrate data on day 4? Where did the .6733 mg/L N go?
My tank water is not zero-carbon, so lets look at the changes (deltas) in nitrogen from day 1 to 3.
and lets look at the sample without Prime to eliminate interference issues.
Looks pretty good until past day 3, when the NO3 production ramps up.
Don't worry, I've got you covered. I'll have NO3 data later, now that NO2 is cleared.
@taricha Nitrate specifically at day 4 though not in the end. The end is valuable too though. Also would of been good if you give look at spectral data during this.
.6733mg N missing from start red line
.7254 mg N missing from start yellow line
You omitted some critical information.
.6733mg N missing from start red line
.7254 mg N missing from start yellow line
You omitted some critical information.
Last edited:
- Joined
- May 22, 2016
- Messages
- 6,542
- Reaction score
- 10,099
unpack this a bit, please. Are you saying Prime would have slowed nitrification at 5+ ppm ammonia, when it doesn't at 1ppm ammonia?@taricha Yes sure this test is at 1.1ppm and .5ppm +/- though. Now test this at levels phd doc says it inhibits growth. +5 ppm.
And I don't know what source you are referring to for that: link?
- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
5 ppm total ammonia does not inhibit the growth of Bio-Spira. Just ran that as one of the treatments in a multi-factorial design experiment. The consumption took longer than 1 ppm but consumption rate appeared to be the same. I have read about ammonia inhibition in freshwater but at higher levels.@taricha Yes sure this test is at 1.1ppm and .5ppm +/- though. Now test this at levels phd doc says it inhibits growth. +5 ppm.
- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
Do you recall what the pH and temperature were when the total ammonia hit 2 ppm?What I can say is that I cycled my very first QT with a clown and Prime. I didn't change the water until ammonia was around 2ppm. I just followed an article online. I understand though that a clown can take a lot. With that said, I have been playing it safe and doing water changes during my TTM and throwing in some Prime for the hell of it. I had to overfeed because my fishes weren't eating initially so had a little ammonia.
- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
Nice demonstration. By the way, why would Prime be involved in cycling an aquarium?One thing that Prime gets wrongfully accused of is slowing a tank cycle.
Some say that since it claims to "bind" ammonia, then the ammonia processing to nitrite and nitrate is slowed by adding Prime. This is incorrect. Here is a demonstration.
I took some tank water, and dosed it to ~1ppm ammonia and split it into three 100mL samples. Two of the three samples got sand from a small cycled tank to process the ammonia.
bottle 1: Tank water + ammonia + Prime (no sand)
bottle 2: Tank water + ammonia (2% sand)
bottle 3: Tank water + ammonia + Prime (2% sand)
left Chart is ammonia testing with API total ammonia. Right chart is Nitrite. Green dashed lines are when Prime was added.

First the ammonia chart. It's quite messy, but it's clear that the samples with sand processed ammonia pretty similarly - perhaps exactly the same, but Prime makes that hard to see. The Prime clearly interfered with the total ammonia test somewhat, and the interference was erratic and unpredictable in the sample without sand. (Perhaps the sand samples broke down the Prime faster and decreased the interfering effect)
On to the Nitrite graph on the right. It's clean and requires almost no explanation. Nitrite starts being produced at the same time, with or without big doses of Prime, similarly it reaches peak NO2 value at the same time and the NO2 gets cleared at the same time - with or without Prime. Neither the bacteria oxidizing Ammonia->NO2 nor those oxidizing NO2->NO3 are slowed in the slightest by repeated big doses of Prime.
So attempts to blame (saltwater) cycling issues on Prime seem totally unfounded. It may make the tests (especially ammonia) harder to interpret - but it doesn't affect the ammonia nitrification process in the slightest. Such explanations from bottle bacteria sellers shouldn't be accepted.
- Joined
- May 22, 2016
- Messages
- 6,542
- Reaction score
- 10,099
To follow up on this data and the question of whether Prime interferes with cycling ammonia to NO3....
here's the final NO3 values....
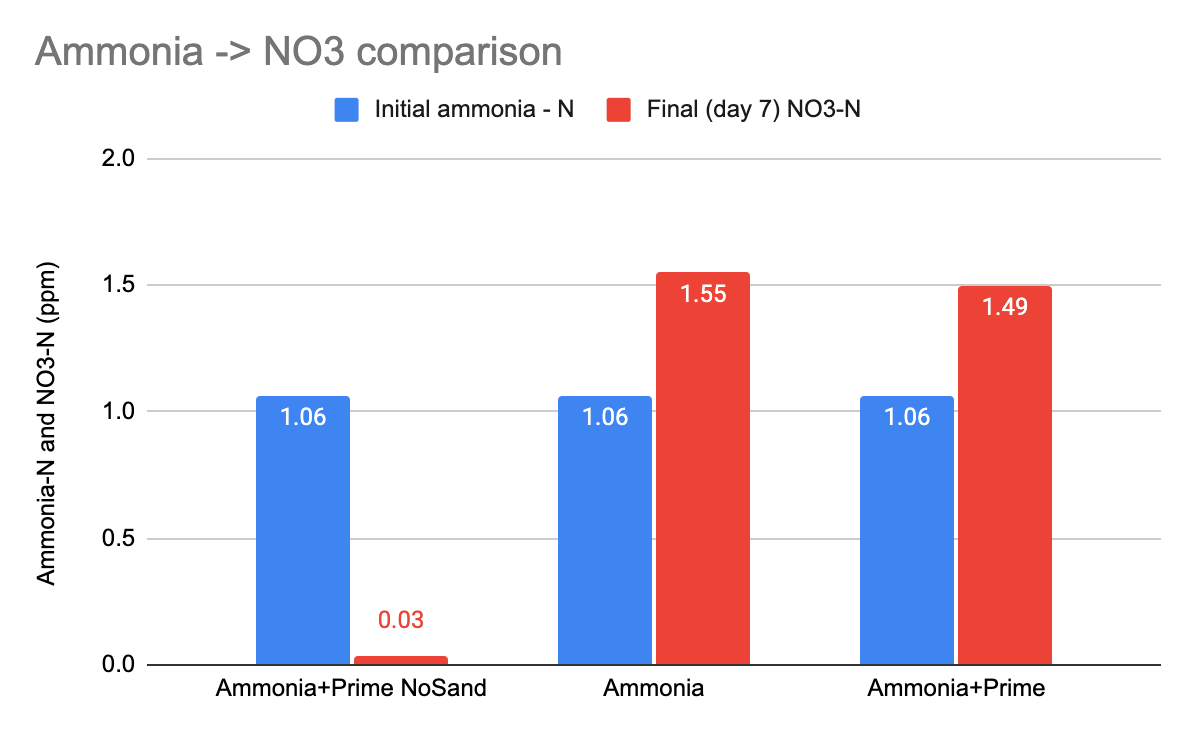
The sample with no sand cycled nothing, and the two samples with sand produced the same amount of NO3 regardless of Prime or not.
So to recap - with or without multiple big doses of Prime, nitrifiers in the sand cycled ammonia to NO2 and finally NO3. They began producing NO2 at the same time, they hit peak NO2 at the same time, they processed away NO2 at the same time, and they generate the same final NO3 values, whether there were Prime doses or not.
here's the final NO3 values....
The sample with no sand cycled nothing, and the two samples with sand produced the same amount of NO3 regardless of Prime or not.
So to recap - with or without multiple big doses of Prime, nitrifiers in the sand cycled ammonia to NO2 and finally NO3. They began producing NO2 at the same time, they hit peak NO2 at the same time, they processed away NO2 at the same time, and they generate the same final NO3 values, whether there were Prime doses or not.
- Joined
- May 22, 2016
- Messages
- 6,542
- Reaction score
- 10,099
Two ways I can think of, either using Prime as a dechlorinator to treat tap water, or if somebody is doing fish-in cycling and gets worried about ammonia.Nice demonstration. By the way, why would Prime be involved in cycling an aquarium?
Apparently Fritz and Dr Tim both warn against this, though this advice may not be saltwater specific.
Fritz
Can I use chemical ammonia removers with FritzZyme® Nitrifying Bacteria?
Chemical ammonia removers work by converting poisonous ammonia to non-toxic compounds. Nitrifying bacteria may not be able to utilize some of these compounds. The use of ammonia removers will only prolong the time needed to establish the biofilter. Repeated use of these chemicals may also lower the pH of the water. Nitrification will cease if the pH drops below 6.5.
Dr. Tim
Do not add ammonia removers to bind the ammonia – overdosing with these products will just increase the cycling time.
I once caused a bacteria bloom because I overdosed prime to prevent ammonia. Cringing when I look back because
1. prime didn’t do anything for ammonia.
2. I didn’t even have ammonia.
3. Bacterial blooms could starve fish (resulting in death) by diminishing oxygen.
1. prime didn’t do anything for ammonia.
2. I didn’t even have ammonia.
3. Bacterial blooms could starve fish (resulting in death) by diminishing oxygen.
- Joined
- Sep 20, 2018
- Messages
- 1,117
- Reaction score
- 1,090
Ammonia monooxygenase is actually not very picky about substrates so it's able to be used for bioremediation. Not sure about the other enzymes though.
I'm not convinced that anything that binds ammonia renders AMO unable to use it as a substrate. Of course, it could be that Prime doesn't bind anything at all...
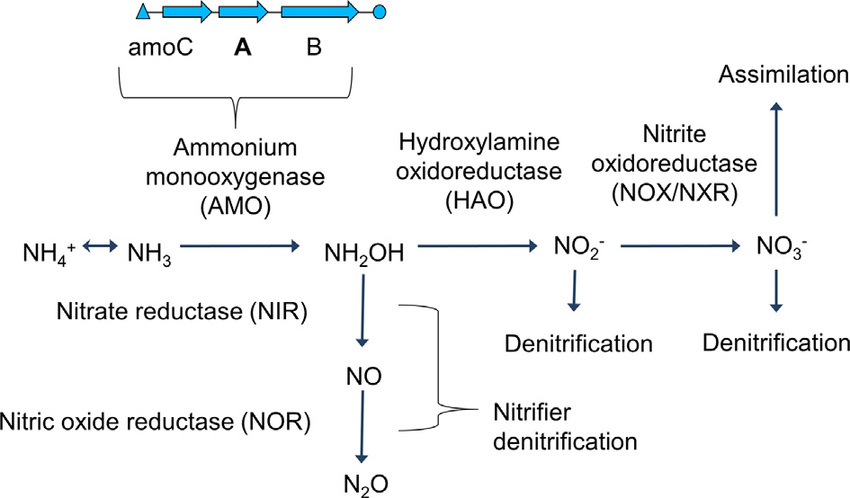
I'm not convinced that anything that binds ammonia renders AMO unable to use it as a substrate. Of course, it could be that Prime doesn't bind anything at all...
- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
Thanks! I might just add this to the list of “UFO’s to use” to stall a cycle.
- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
When you say that AMO is not picky about substates, does that mean it can oxidize methylamine?Ammonia monooxygenase is actually not very picky about substrates so it's able to be used for bioremediation. Not sure about the other enzymes though.
I'm not convinced that anything that binds ammonia renders AMO unable to use it as a substrate. Of course, it could be that Prime doesn't bind anything at all...

- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
How did Prime use cause a bacteria bloom? was it like dosing vinegar?I once caused a bacteria bloom because I overdosed prime to prevent ammonia. Cringing when I look back because
1. prime didn’t do anything for ammonia.
2. I didn’t even have ammonia.
3. Bacterial blooms could starve fish (resulting in death) by diminishing oxygen.
@Malcontent This doesn't actually prove if it is bound or not.
@taricha Not saying prime but the actual claim that intermediates in the process cause slowing.
Still missing .52138 mg/L N red line test
Still missing .53511 mg/L N yellow line test
Missing from starting nitrogen. Where did it go?
This is why an over lay of nitrate at same time, and over lay of spectral would of been brilliant.
6 days does seem pretty slow though. I guess it would be hard to recreate a tank in a vial. Usually see this being processed in less days in tank setting at these levels. If one goes with 1 lb rock per gallon. Not including sand this is around 11% not 2%. I wonder how much faster it would happen. Also wonder about comment above +5ppm. Ranges that claim to inhibit process.
@taricha Not saying prime but the actual claim that intermediates in the process cause slowing.
Still missing .52138 mg/L N red line test
Still missing .53511 mg/L N yellow line test
Missing from starting nitrogen. Where did it go?
This is why an over lay of nitrate at same time, and over lay of spectral would of been brilliant.
6 days does seem pretty slow though. I guess it would be hard to recreate a tank in a vial. Usually see this being processed in less days in tank setting at these levels. If one goes with 1 lb rock per gallon. Not including sand this is around 11% not 2%. I wonder how much faster it would happen. Also wonder about comment above +5ppm. Ranges that claim to inhibit process.
Yes. Cloudy water.How did Prime use cause a bacteria bloom? was it like dosing vinegar?
Similar threads
- Replies
- 2
- Views
- 73
- Replies
- 29
- Views
- 288
- Replies
- 38
- Views
- 690
- Replies
- 58
- Views
- 2,655

















