Bentonite clay is an aluminum silicate. A zeolite.baked clay is not zeolite, zeolite is a specific type of rock mainly made of aluminum
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dr. Kevin Novak Anoxic Filtration System.
- Thread starter dryworm
- Start date
- Tagged users None
imo put it in a place with no light and also be sure it gets ambient flow on all sides (two issues with my setup)I'll be setting up a BCB in this newly cycled 10 gallon for experimenting with. Any suggestions or ideas for what else yall would like to see happen here?
Probably won't be able to do the no light with this set up but definitely something I would like to try. It will be set up with flow all around it. I'm going to remove the box filter and will just have the bcb and the power head in the tank for filtration. I will pull out some of the rocks as well. There is also a very thin layer of finely crushed aragonite for substrate. Should be able to have the bcb set up tomorrow. Just waiting for the craft mesh to come in.imo put it in a place with no light and also be sure it gets ambient flow on all sides
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,349
- Reaction score
- 63,691
I'm surprised we are still talking about this.
Is anyone suggesting this process is any different than the denitrification processes that reefers have been using for many decades?
Is anyone suggesting this process is any different than the denitrification processes that reefers have been using for many decades?
I'm surprised we are still talking about this.
Is anyone suggesting this process is any different than the denitrification processes that reefers have been using for many decades?
Personally I've only been in the hobby and subsequent science since 2017/2018, but what I notice most is that 90% or more of reefers do not use the techniques that Novak uses: basically floating deep sand beds in the basket application, and even plenums are hardly used anymore (with or without flow). I know there was the study about deep sand bed effectiveness which arguably settled the question of DSB usefulness in reef aquaria but I recall there were a few questions the study did not answer, namely providing flow under a plenum DSB combination. Also, Novak specifies the substrate to use in his design, which contrasts with most reef substrates (importantly, non-carbonate rock).
I suppose it boils down to his baskets being more effective than the widely available dry rock at providing deep spaces where the anoxic zones can form and interact with the tank water. After all, phosphates can't adsorb in bentonite clay or zeolite, and the hydrodynamics of rubble is logically better than solid carbonate rock.
I guess the specific question here is, what is the goal of Dr. Novak's system? Is it just to support denitrification, or something else as well?Personally I've only been in the hobby and subsequent science since 2017/2018, but what I notice most is that 90% or more of reefers do not use the techniques that Novak uses: basically floating deep sand beds in the basket application, and even plenums are hardly used anymore (with or without flow). I know there was the study about deep sand bed effectiveness which arguably settled the question of DSB usefulness in reef aquaria but I recall there were a few questions the study did not answer, namely providing flow under a plenum DSB combination. Also, Novak specifies the substrate to use in his design, which contrasts with most reef substrates (importantly, non-carbonate rock).
I suppose it boils down to his baskets being more effective than the widely available dry rock at providing deep spaces where the anoxic zones can form and interact with the tank water. After all, phosphates can't adsorb in bentonite clay or zeolite, and the hydrodynamics of rubble is logically better than solid carbonate rock.
be the main source of freshwater high bio-load nitrate reduction, in my wordsI guess the specific question here is, what is the goal of Dr. Novak's system? Is it just to support denitrification, or something else as well?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,349
- Reaction score
- 63,691
Personally I've only been in the hobby and subsequent science since 2017/2018, but what I notice most is that 90% or more of reefers do not use the techniques that Novak uses: basically floating deep sand beds in the basket application, and even plenums are hardly used anymore (with or without flow). I know there was the study about deep sand bed effectiveness which arguably settled the question of DSB usefulness in reef aquaria but I recall there were a few questions the study did not answer, namely providing flow under a plenum DSB combination. Also, Novak specifies the substrate to use in his design, which contrasts with most reef substrates (importantly, non-carbonate rock).
I suppose it boils down to his baskets being more effective than the widely available dry rock at providing deep spaces where the anoxic zones can form and interact with the tank water. After all, phosphates can't adsorb in bentonite clay or zeolite, and the hydrodynamics of rubble is logically better than solid carbonate rock.
There are as many different embodiments of heterotrophic denitrification in reef tanks as there are models of cars. I don't see this as particularly different than any of these others. They generally all work to some extent.
There are loads of different commercial media designed to accomplish denitrification (e.g., siporax, Marinepure, etc.) as well as different organics that boost the process (NOPOX, vinegar, vodka, biopellets, etc.) and some more sophisticated devices to facilitate it (carbon denitrators). And, of course, there has been decades discussion of the best way to use sand beds and porous live rock to accomplish this, such as reverse flow, plenums, remote sand beds, slow flow, fast flow, different depths, etc.)
If anyone thinks this particular way of accomplishing denitritification in a reef tank is better than these others, that's a fine opinion to express and some experimental results could be interesting.
But I really do not see that this is something new or different.
I'm not seeing a concern with phosphate binding to substrates, but if that did concern someone, using silica sand eliminates it.
Its a box filled with cat litter. Pretty novel if you ask me. Just the cost aspect and ease of maintenance is a big difference from other methods.There are as many different embodiments of heterotrophic denitrification in reef tanks as there are models of cars. I don't see this as particularly different than any of these others. They generally all work to some extent.
There are loads of different commercial media designed to accomplish denitrification (e.g., siporax, Marinepure, etc.) as well as different organics that boost the process (NOPOX, vinegar, vodka, biopellets, etc.) and some more sophisticated devices to facilitate it (carbon denitrators). And, of course, there has been decades discussion of the best way to use sand beds and porous live rock to accomplish this, such as reverse flow, plenums, remote sand beds, slow flow, fast flow, different depths, etc.)
If anyone thinks this particular way of accomplishing denitritification in a reef tank is better than these others, that's a fine opinion to express and some experimental results could be interesting.
But I really do not see that this is something new or different.
I'm not seeing a concern with phosphate binding to substrates, but if that did concern someone, using silica sand eliminates it.
Why would this method need to be the best to be practical? People use roller mats and filter socks and they do the same thing.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,349
- Reaction score
- 63,691
Its a box filled with cat litter. Pretty novel if you ask me. Just the cost aspect and ease of maintenance is a big difference from other methods.
Why would this method need to be the best to be practical? People use roller mats and filter socks and they do the same thing.
Nothing needs to be perfect. All it needs is someone to want to use it.
I'm personally see no reason to use in my system as I think other methods offer other benefits that make them a better choice (organic carbon dosing, for example, provides food for filter feeders).
I personally would not EVER put commercial kitty litter in my tank, any more than I would use stump remover from Home Depot for dosing nitrate without testing it carefully first. That doesn't stop others from doing it.
Claims it cannot dissolve are, IMO, not substantiated.
Roller mats and filter socks most definitely do not accomplish the exact same thing. They export particulates. While I do not use them either, they have a benefit that goes beyond nutrient levels.
I meant that roller mats and filter socks are similar like DSB and a box of kitty litter is similar.Nothing needs to be perfect. All it needs is someone to want to use it.
I'm personally see no reason to use in my system as I think other methods offer other benefits that make them a better choice (organic carbon dosing, for example, provides food for filter feeders).
I personally would not EVER put commercial kitty litter in my tank, any more than I would use stump remover from Home Depot for dosing nitrate without testing it carefully first. That doesn't stop others from doing it.
Claims it cannot dissolve are, IMO, not substantiated.
Roller mats and filter socks most definitely do not accomplish the exact same thing. They export particulates. While I do not use them either, they have a benefit that goes beyond nutrient levels.
Everything dissolves with enough time so yea prob.
I actually just thought about the other huge benefit of this filtration method. Dr. Novak claims to have developed this method for use with koi ponds initially and he claims there’s not better filtration system for the purpose and will trust thousands of dollars of koi to it. If this is true you could make a reef tank the size of koi pond using a few biocenocis baskets. Seems pretty cool.
Hey Randy, I think the confusion on why this might be better is in how the system works in freshwater and why it is different then a standard 'deep sand bed' type system.There are as many different embodiments of heterotrophic denitrification in reef tanks as there are models of cars. I don't see this as particularly different than any of these others. They generally all work to some extent.
There are loads of different commercial media designed to accomplish denitrification (e.g., siporax, Marinepure, etc.) as well as different organics that boost the process (NOPOX, vinegar, vodka, biopellets, etc.) and some more sophisticated devices to facilitate it (carbon denitrators). And, of course, there has been decades discussion of the best way to use sand beds and porous live rock to accomplish this, such as reverse flow, plenums, remote sand beds, slow flow, fast flow, different depths, etc.)
If anyone thinks this particular way of accomplishing denitritification in a reef tank is better than these others, that's a fine opinion to express and some experimental results could be interesting.
But I really do not see that this is something new or different.
I'm not seeing a concern with phosphate binding to substrates, but if that did concern someone, using silica sand eliminates it.
Basically the idea is that going beyond a standard filtration method you set up a positive ion 'trap'. Basically the idea is that the clay zones ABC, and the high iron laterite core of the biocenosis basket will draw ammonium ions in.
And since this zone will occur, that the filter 'can't clog' because it's not filtering particles, but drawing ammonium in which will be then consumed. The aspect of this filter as written is to provide nitrifying on the aerobic exterior zones and then denitrifying within the laterite compartment/anoxic zone.
What I tried to point out is that you can't create this charged zone in saltwater as the high concentration of sodium and potassium ions will eliminate the negatively charged substrate with ease. Thus no ammonium draw which means the system as described can't work in saltwater.
Will it have surface area to provide help? Sure but just like you have already mentioned so will a box of rocks.
Not sure how you would even go about testing a system which we know will provide some nitrification/denitrification when the premise (in saltwater) is flawed?
Pretty interesting idea for freshwater though, especially if you do like to grow plants within the boxes.
If what you are saying is true then wouldn’t that mean that no ammonium absorbing materials would work in salt water?Hey Randy, I think the confusion on why this might be better is in how the system works in freshwater and why it is different then a standard 'deep sand bed' type system.
Basically the idea is that going beyond a standard filtration method you set up a positive ion 'trap'. Basically the idea is that the clay zones ABC, and the high iron laterite core of the biocenosis basket will draw ammonium ions in.
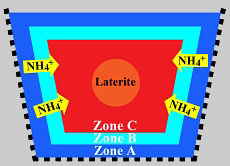
And since this zone will occur, that the filter 'can't clog' because it's not filtering particles, but drawing ammonium in which will be then consumed. The aspect of this filter as written is to provide nitrifying on the aerobic exterior zones and then denitrifying within the laterite compartment/anoxic zone.
What I tried to point out is that you can't create this charged zone in saltwater as the high concentration of sodium and potassium ions will eliminate the negatively charged substrate with ease. Thus no ammonium draw which means the system as described can't work in saltwater.
Will it have surface area to provide help? Sure but just like you have already mentioned so will a box of rocks.
Not sure how you would even go about testing a system which we know will provide some nitrification/denitrification when the premise (in saltwater) is flawed?
Pretty interesting idea for freshwater though, especially if you do like to grow plants within the boxes.
If what you are saying is true then wouldn’t that mean that no ammonium absorbing materials would work in salt water?
Yes that is true. One company tried to pass off their zeolites as being able to accomplish this. I believe they don't say that anymore?? Not sure. The problem is that the clays/zeolites usually have ion preference. Sodium and potassium tend to adsorb better, and when you consider that absolute concentration differences ammonium will never stand a chance in saltwater. Interesting anecdotal evidence also suggests that some zeolites have a stronger potassium capability and while sodium is in much higher concentration, the aquarists who use these zeolites struggle with low potassium issues (and also some people have suggested they crashed their tank by quickly lowering their potassium by adding a large amount of zeolites at one time). The companies who market zeolites make sure to suggest adding small amounts at a time.
Believe me when I say that the medical and molecular biology industries would absolutely go gaga over an ammonium specific adsorbing material. It would save lives and would improve many more lives. And so I truly hope someone does figure this out in the future.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,349
- Reaction score
- 63,691
Hey Randy, I think the confusion on why this might be better is in how the system works in freshwater and why it is different then a standard 'deep sand bed' type system.
Basically the idea is that going beyond a standard filtration method you set up a positive ion 'trap'. Basically the idea is that the clay zones ABC, and the high iron laterite core of the biocenosis basket will draw ammonium ions in.

And since this zone will occur, that the filter 'can't clog' because it's not filtering particles, but drawing ammonium in which will be then consumed. The aspect of this filter as written is to provide nitrifying on the aerobic exterior zones and then denitrifying within the laterite compartment/anoxic zone.
What I tried to point out is that you can't create this charged zone in saltwater as the high concentration of sodium and potassium ions will eliminate the negatively charged substrate with ease. Thus no ammonium draw which means the system as described can't work in saltwater.
Will it have surface area to provide help? Sure but just like you have already mentioned so will a box of rocks.
Not sure how you would even go about testing a system which we know will provide some nitrification/denitrification when the premise (in saltwater) is flawed?
Pretty interesting idea for freshwater though, especially if you do like to grow plants within the boxes.
Thanks. I was not following it that closely.
IMO, zeovit incorrectly claimed this in the past (don't know if they still do) for their zeostones "attracting" ammonia by binding it, and then making it more available to local bacteria. IMO, that is flawed thinking, and the bioavailabiltiy of ammonia is reduced near the stones, not increased.
They just did not get the cocnept that
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,349
- Reaction score
- 63,691
If what you are saying is true then wouldn’t that mean that no ammonium absorbing materials would work in salt water?
Some materials can bind ammonia, in seawater and fresh, and that makes it necessarily less bioavailable.
Odd, IMO, that somehow folks mistakenly think that binding it in the molecularly narrow pores of a zeolite (where bacteria cannot possibly go) and removing it from solution where bacteria can get it somehow makes it more bioavailable.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,349
- Reaction score
- 63,691
Yes that is true. One company tried to pass off their zeolites as being able to accomplish this. I believe they don't say that anymore?? Not sure. The problem is that the clays/zeolites usually have ion preference. Sodium and potassium tend to adsorb better, and when you consider that absolute concentration differences ammonium will never stand a chance in saltwater. Interesting anecdotal evidence also suggests that some zeolites have a stronger potassium capability and while sodium is in much higher concentration, the aquarists who use these zeolites struggle with low potassium issues (and also some people have suggested they crashed their tank by quickly lowering their potassium by adding a large amount of zeolites at one time). The companies who market zeolites make sure to suggest adding small amounts at a time.
Believe me when I say that the medical and molecular biology industries would absolutely go gaga over an ammonium specific adsorbing material. It would save lives and would improve many more lives. And so I truly hope someone does figure this out in the future.
I was on the scientific advisory board of a company trying to bind ammonia in the GI tract of certain patients. Not an easy thing to accomplish at the amounts needed at the available concentrations. They have since been bought out by a bigger company that didn't want to continue pursuing that project.
I was on the scientific advisory board of a company trying to bind ammonia in the GI tract of certain patients. Not an easy thing to accomplish at the amounts needed at the available concentrations. They have since been bought out by a bigger company that didn't want to continue pursuing that project.
Cool! Thank you for the correction about ammonia binders and the update on the science.
I wonder if that company ever considered that ammonia is a big issue in cell culture as well. The cell culture market is absolutely huge, especially with the newer vaccine manufacturing procedures.
Similar threads
- Replies
- 42
- Views
- 1,884
- Replies
- 3,079
- Views
- 91,751
- Replies
- 18
- Views
- 1,508
- Replies
- 490
- Views
- 16,354

















