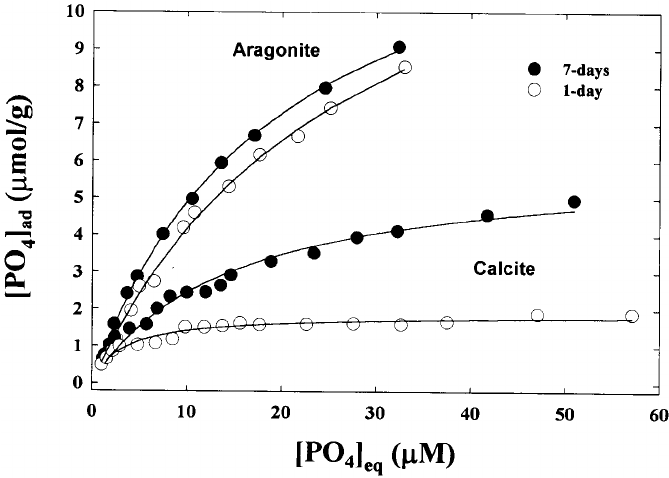
Hey JDA,This is technical, but it might help in your quest. po4 does not leach out of rock. It unbinds. This might seem the same, but binding has rules and is predictable. The more than you raise po4 in the water, the more the rock/sand binds. The rock and sand will not just release/leach po4 - you have to lower the water column level of po4 for the rock/sand to unbind.
This is a bind and unbind type of deal, not absorb and leach. You can plan an approach to this and the results will follow.
Growing algae will have rules too. Pay attention to the people with their own chemistry forums and folks who have run some of the largest captive reefs on the planet... you cannot remove algae from one place by growing algae in another. Period. Step up your algae consumers in the tank and if you do grow algae in other places without consumers, your waste products can be lower. Usually there are easy answers, just people who do not know how to read them. Please spend a bit on algae consumers or not much will change.
I find this interesting. Please help educate me a bit more. Before reading your post, I assumed on R2R the term bind meant absorb and the term unbind meant leach. What's the difference?
Thanks,