- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
How well do hobby alkalinity measurements detect the alkalinity increase from adding Boost pH+ to a saltwater aquarium? I compare the results of the Hanna Checker with the Red Sea and API titration methods.
Alkalinity in our aquarium water is usually determined by titration, the addition of acid to a sample of aquarium water containing an indicator until a change in color occurs at the endpoint. The amount of acid needed to cause the indicator to change color corresponds to the amount of alkalinity present in the sample.
The Hanna alkalinity Checker uses a somewhat different approach, adding a single dose of acid and measuring the resulting pH with a pH sensitive dye. The dye color intensity (absorbance measured at 606 nm) increases with increasing pH.
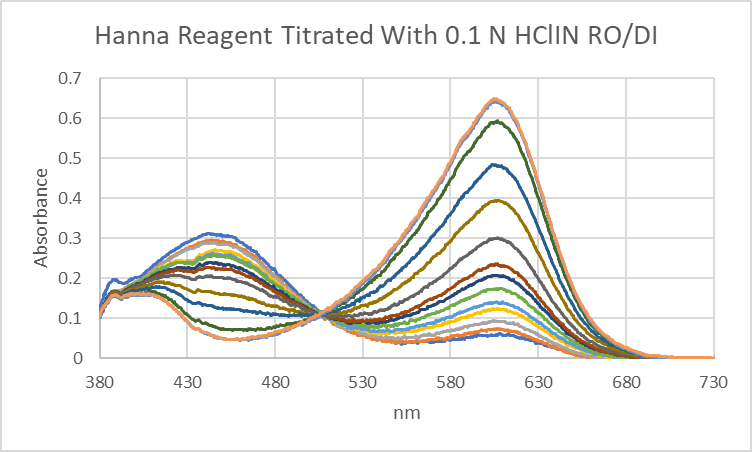
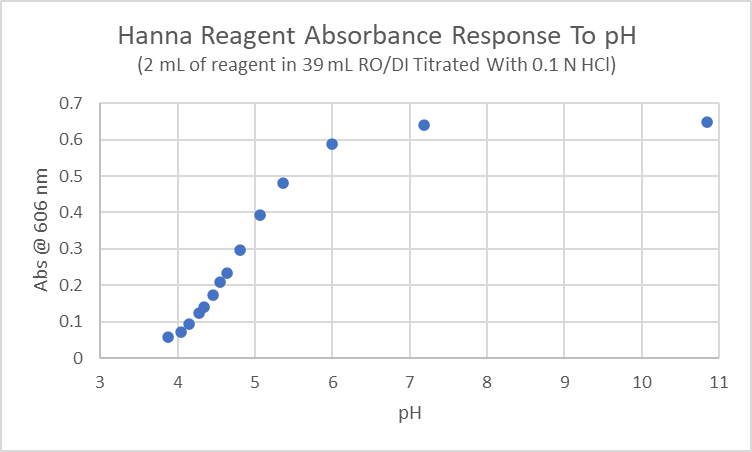
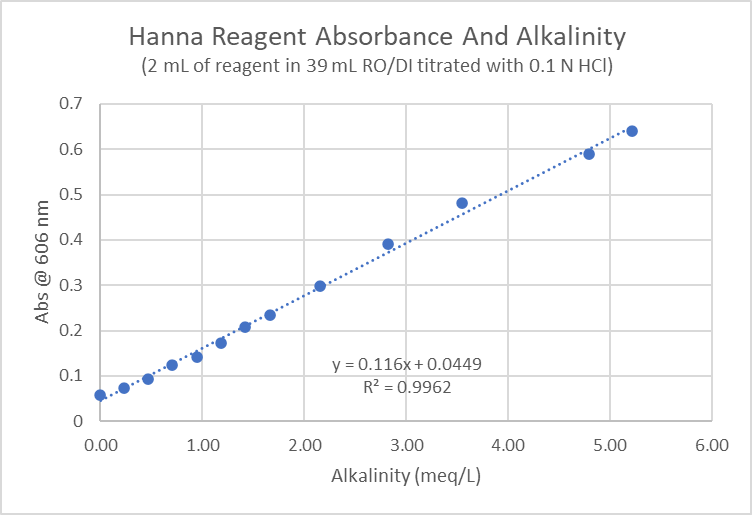
A higher alkalinity in the sample causes a higher pH in the reagent sample mixture. Alkalinity and color intensity are linearly related.
Methods Modification
API. This titration method adds acid in large steps, making the detection of small changes in alkalinity impossible. To correct this with a minimum amount of change to the API method, a back titration strategy was used. In this approach, one additional drop of the API reagent is added after the endpoint is reached. Then using a one mL syringe fitted with a tip, additional water sample is added drop wise to the over titrated sample until the endpoint color reappears. Multiplying the number of reagent drops (alkalinity) by 5 and dividing by the total volume of the sample, i.e. 5 mL plus the extra volume, gives the alkalinity of the sample with higher precision.
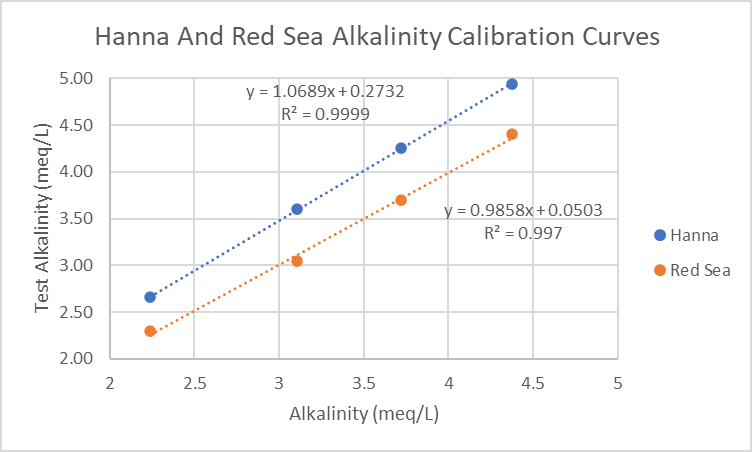
Hanna Checker. When I compared my Hanna Checker alkalinity measurements to the amount measured by titration, the Checker results were consistently higher (post #170). To correct the Checker readings, a calibration curve was generated with a sodium bicarbonate solution and titrated aquarium water. Note how well the Red Sea titration performs.
Measuring Alkalinity Change.
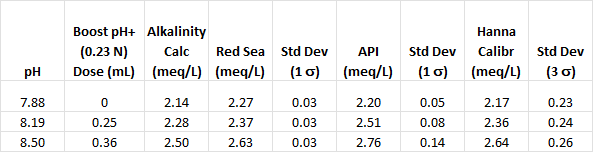
The alkalinity of a 500 mL sample aquarium water was determined by titration (see table, first entry in “Alkalinity Calc” column) and the three hobby kits. Then a sufficient amount of Boost pH+ (0.23 N, 230 meq/L) was added to increase the pH 0.3 units, followed by alkalinity measurements. A total of two Boost pH+ additions were made. Triplicate measurements were made with Red Sea and API, and one measurement with the Hanna Checker (precision is calculated) after each Boost pH+ addition. The calculated alkalinity of the solution is the sum of the initial alkalinity and the added Boost pH+ alkalinity. The results are summarized in the table below and the bar chart below the table provides a visual comparison.
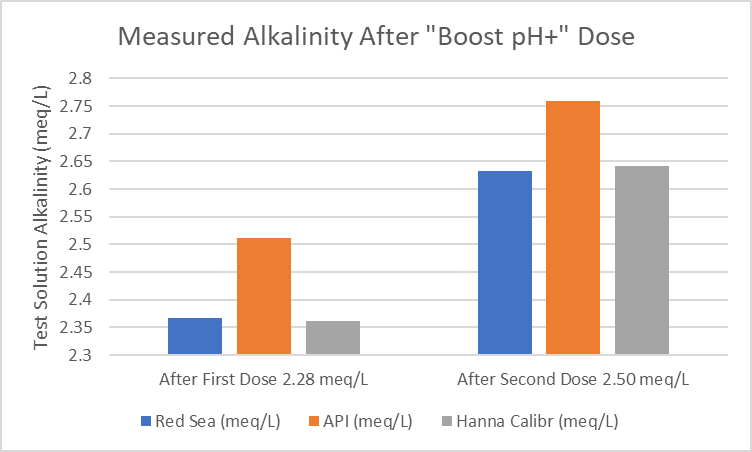
I conclude that all methods detect an alkalinity increase when Boost pH+ is added to aquarium water and provide reasonably accurate measurements with decent precision. The API method might be improved by moving to a syringe titration because of variable drop size using the bottle and the high concentration of the reagent. In both titrations kits, the endpoint color was aggravatingly vague to my eye but with practice it would probably be very repeatable. The Hanna high reading is a mystery. I need to buy another lot of reagent and measure both the acid content and dye concentration to see if either or both are different than the lot of reagent I now have.
Alkalinity in our aquarium water is usually determined by titration, the addition of acid to a sample of aquarium water containing an indicator until a change in color occurs at the endpoint. The amount of acid needed to cause the indicator to change color corresponds to the amount of alkalinity present in the sample.
The Hanna alkalinity Checker uses a somewhat different approach, adding a single dose of acid and measuring the resulting pH with a pH sensitive dye. The dye color intensity (absorbance measured at 606 nm) increases with increasing pH.
A higher alkalinity in the sample causes a higher pH in the reagent sample mixture. Alkalinity and color intensity are linearly related.
Methods Modification
API. This titration method adds acid in large steps, making the detection of small changes in alkalinity impossible. To correct this with a minimum amount of change to the API method, a back titration strategy was used. In this approach, one additional drop of the API reagent is added after the endpoint is reached. Then using a one mL syringe fitted with a tip, additional water sample is added drop wise to the over titrated sample until the endpoint color reappears. Multiplying the number of reagent drops (alkalinity) by 5 and dividing by the total volume of the sample, i.e. 5 mL plus the extra volume, gives the alkalinity of the sample with higher precision.
Hanna Checker. When I compared my Hanna Checker alkalinity measurements to the amount measured by titration, the Checker results were consistently higher (post #170). To correct the Checker readings, a calibration curve was generated with a sodium bicarbonate solution and titrated aquarium water. Note how well the Red Sea titration performs.
Measuring Alkalinity Change.
The alkalinity of a 500 mL sample aquarium water was determined by titration (see table, first entry in “Alkalinity Calc” column) and the three hobby kits. Then a sufficient amount of Boost pH+ (0.23 N, 230 meq/L) was added to increase the pH 0.3 units, followed by alkalinity measurements. A total of two Boost pH+ additions were made. Triplicate measurements were made with Red Sea and API, and one measurement with the Hanna Checker (precision is calculated) after each Boost pH+ addition. The calculated alkalinity of the solution is the sum of the initial alkalinity and the added Boost pH+ alkalinity. The results are summarized in the table below and the bar chart below the table provides a visual comparison.
I conclude that all methods detect an alkalinity increase when Boost pH+ is added to aquarium water and provide reasonably accurate measurements with decent precision. The API method might be improved by moving to a syringe titration because of variable drop size using the bottle and the high concentration of the reagent. In both titrations kits, the endpoint color was aggravatingly vague to my eye but with practice it would probably be very repeatable. The Hanna high reading is a mystery. I need to buy another lot of reagent and measure both the acid content and dye concentration to see if either or both are different than the lot of reagent I now have.