Last edited:
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Experimental testing of Brightwell Boost pH +
- Thread starter Randy Holmes-Farley
- Start date
- Tagged users None
Me too, but for a different reason.
My what the heck moment happened while titrating. Are we at the point of needing to prove first principles all over again to squelch vendor claims?
Yes. 100% yes. Keep up the good work guys! This is fantastic.
Thanks very much for doing this experiment!
Any chance we can get a Red Sea alk test on hydroxide with a similar alk rise or pH rise as the Boost pH+?
Sorry to seem demanding, but the more evidence we have, the easier it will be to convince Jack.
If Jack needs any more convincing, it’s a lost cause
- Joined
- May 22, 2016
- Messages
- 6,559
- Reaction score
- 10,130
Here it is with MiamiReef's NaOH comparison to Boost on Red Sea alk test. (The yellow data now is a paired test).
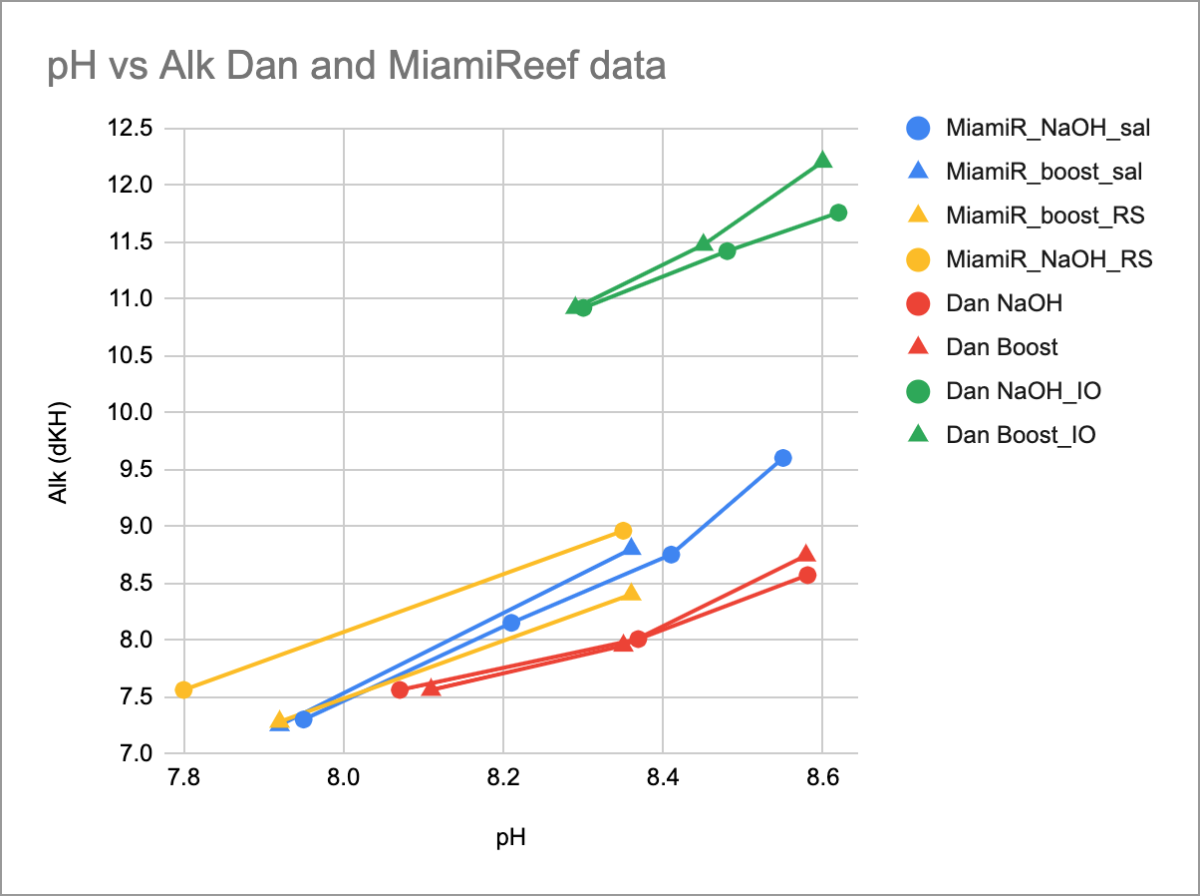
The yellow lines start at different points because the tank water had slightly different pH and alk on different days, nonetheless the trend is again almost identical for NaOH and Boost pH+ additions. Boost pH+ still adds at least as much alk as NaOH for the same pH increase.
The yellow lines start at different points because the tank water had slightly different pH and alk on different days, nonetheless the trend is again almost identical for NaOH and Boost pH+ additions. Boost pH+ still adds at least as much alk as NaOH for the same pH increase.
Ya. I raised my tank’s alk a bit last night after yesterday‘s experiment lol.Here it is with MiamiReef's NaOH comparison to Boost on Red Sea alk test. (The yellow data now is a paired test).

The yellow lines start at different points because the tank water had slightly different pH and alk on different days, nonetheless the trend is again almost identical for NaOH and Boost pH+ additions. Boost pH+ still adds at least as much alk as NaOH for the same pH increase.
The most enlightening thing after this whole debacle is how important it is to recalibrate your pH probes regularly. I thought my pH was 8.25, but when I cleaned and recalibrated it, it was actually 7.8.
I recalibrated it again because it had to be an error. Same results. I then ordered new pH solutions: same results: 7.8
So ya, this experiment was very enlightening for me.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,431
- Reaction score
- 63,793
You guys all did a great job!
I think we are all set now. I’ll send that last graph to Jack and will work on a summary post/ thread when I get a chance.
The Boost pH+ product looks to raise pH and alk just as one would expect if its only active ingredient was the hydroxide known from Brightwell to be in it. There’s no need to invoke or hypothesize any effects from proprietary ingredients.
As an aside, the expected pH boost from adding hydroxide (or carbonate) depends on both the starting alk and the starting pH. The effect is biggest at low starting alk and pH for reasons related to buffering detailed here:
 reefs.com
reefs.com
Buffering capacity can be quantified using the buffer intensity, b, defined mathematically in a way that is easy to calculate, but that isn’t worth detailing here.2 The units of the buffering intensity can be expressed as meq/L or meq/L/pH unit (these are equivalent since pH is really a dimensionless parameter). Thinking about it as meq/L/pH unit makes it easier to understand that it is a measure of the amount of alkalinity (or acidity; either one measured as meq/L) that needs to be added to impact the pH up or down by one unit (though that is a substantial simplification).
In the case of normal seawater at pH 8.2, b = 0.19 meq/L/pH unit for the boric acid/borate system, and 0.63 meq/L/pH unit for the bicarbonate/carbonate system. These values are additive, and result in a total buffering of b = 0.82 meq/L/pH unit. Under these conditions, the boric acid/borate system provides about 23% of the total buffering, while the bicarbonate/carbonate system provides about 77%.
If the pH of normal seawater is raised to 8.5, the total buffering is b = 1.2 meq/L/pH unit, or about 40% greater than at pH 8.2 (because both systems are closer to the pKa). At this pH, the relative contribution of the two systems to the total capacity is only slightly different than at pH 8.2, with 20% from borate and 80% from carbonate.
If the pH of normal seawater is lowered to 7.8, the total buffering is b = 0.42 meq/L/pH unit, or about half that at pH 8.2 (because both systems are farther from the pKa). At this pH, the relative contribution of the two systems to the total capacity is also only slightly different than at pH 8.2, with 29% from borate and 71% from carbonate.
I think we are all set now. I’ll send that last graph to Jack and will work on a summary post/ thread when I get a chance.
The Boost pH+ product looks to raise pH and alk just as one would expect if its only active ingredient was the hydroxide known from Brightwell to be in it. There’s no need to invoke or hypothesize any effects from proprietary ingredients.
As an aside, the expected pH boost from adding hydroxide (or carbonate) depends on both the starting alk and the starting pH. The effect is biggest at low starting alk and pH for reasons related to buffering detailed here:

Chemistry And The Aquarium: Boron In A Reef Tank
This article also discusses the sources and sinks for boron in reef tanks, and shows that while typical tanks may be slightly depleted in boron with respect to natural levels, most are not so significantly depleted that any correction is required. Nevertheless, how to test for and supplement...
Buffering capacity can be quantified using the buffer intensity, b, defined mathematically in a way that is easy to calculate, but that isn’t worth detailing here.2 The units of the buffering intensity can be expressed as meq/L or meq/L/pH unit (these are equivalent since pH is really a dimensionless parameter). Thinking about it as meq/L/pH unit makes it easier to understand that it is a measure of the amount of alkalinity (or acidity; either one measured as meq/L) that needs to be added to impact the pH up or down by one unit (though that is a substantial simplification).
In the case of normal seawater at pH 8.2, b = 0.19 meq/L/pH unit for the boric acid/borate system, and 0.63 meq/L/pH unit for the bicarbonate/carbonate system. These values are additive, and result in a total buffering of b = 0.82 meq/L/pH unit. Under these conditions, the boric acid/borate system provides about 23% of the total buffering, while the bicarbonate/carbonate system provides about 77%.
If the pH of normal seawater is raised to 8.5, the total buffering is b = 1.2 meq/L/pH unit, or about 40% greater than at pH 8.2 (because both systems are closer to the pKa). At this pH, the relative contribution of the two systems to the total capacity is only slightly different than at pH 8.2, with 20% from borate and 80% from carbonate.
If the pH of normal seawater is lowered to 7.8, the total buffering is b = 0.42 meq/L/pH unit, or about half that at pH 8.2 (because both systems are farther from the pKa). At this pH, the relative contribution of the two systems to the total capacity is also only slightly different than at pH 8.2, with 29% from borate and 71% from carbonate.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,431
- Reaction score
- 63,793
I’m in the process of the experiment. One thing’s for sure: I need much more Brightwell boost pH + to get the pH jump than the 1N NaOH.
Related to that comment, Dan’s direct titration of the product would indicate it would take about 4x as much of the Boost pH to match 1 N NaOH:
“Titrated Boost pH+ this morning and it is 0.23 N”
- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
Check out plot in post #115. My data says an equivalent of alkalinity of Boost pH+ has the same effect on pH as an equivalent of alkalinity of NaOH regardless of the starting alkalinity.Related to that comment, Dan’s direct titration of the product would indicate it would take about 4x as much of the Boost pH to match 1 N NaOH:
“Titrated Boost pH+ this morning and it is 0.23 N”
By the way, I ordered a Red Sea alkalinity kit and plan to relook at the alkalinity measurements with it, the Hanna Checker and titration with a pH meter. Additives will be Boost pH+, sodium acetate, sodium bicarbonate and sodium hydroxide. I will monitor color changes with a spectrophotometer along with pH to remove color perception from end point determination. Data collection will be complete after Christmas. My hypothesis is that I don’t have a firm grip on the science
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,431
- Reaction score
- 63,793
Check out plot in post #115. My data says an equivalent of alkalinity of Boost pH+ has the same effect on pH as an equivalent of alkalinity of NaOH regardless of the starting alkalinity.
By the way, I ordered a Red Sea alkalinity kit and plan to relook at the alkalinity measurements with it, the Hanna Checker and titration with a pH meter. Additives will be Boost pH+, sodium acetate, sodium bicarbonate and sodium hydroxide. I will monitor color changes with a spectrophotometer along with pH to remove color perception from end point determination. Data collection will be complete after Christmas. My hypothesis is that I don’t have a firm grip on the science
Sounds great!
- Joined
- May 22, 2016
- Messages
- 6,559
- Reaction score
- 10,130
There's an aspect of what you are looking at that I think is really interesting.Check out plot in post #115. My data says an equivalent of alkalinity of Boost pH+ has the same effect on pH as an equivalent of alkalinity of NaOH regardless of the starting alkalinity.
By the way, I ordered a Red Sea alkalinity kit and plan to relook at the alkalinity measurements with it, the Hanna Checker and titration with a pH meter. Additives will be Boost pH+, sodium acetate, sodium bicarbonate and sodium hydroxide. I will monitor color changes with a spectrophotometer along with pH to remove color perception from end point determination. Data collection will be complete after Christmas. My hypothesis is that I don’t have a firm grip on the science
I wonder if you make a solution of the hanna alk reagent in saltwater and look at the color vs pH - does some ingredient in Boost pH cause the hanna alk color to vary from its normal ph-based color.
I've also heard one or two people say that Prime boosts their hanna alk result.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,431
- Reaction score
- 63,793
There's an aspect of what you are looking at that I think is really interesting.
I wonder if you make a solution of the hanna alk reagent in saltwater and look at the color vs pH - does some ingredient in Boost pH cause the hanna alk color to vary from its normal ph-based color.
I've also heard one or two people say that Prime boosts their hanna alk result.
What is the dye in the Hanna? Maybe it gets chemically reduced by Prime.
- Joined
- May 22, 2016
- Messages
- 6,559
- Reaction score
- 10,130
I don't actually have a good grasp on how hanna alk works, beyond your general explanations of what it likely does.What is the dye in the Hanna? Maybe it gets chemically reduced by Prime.
- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
Yeah, that would be interesting! Will do.There's an aspect of what you are looking at that I think is really interesting.
I wonder if you make a solution of the hanna alk reagent in saltwater and look at the color vs pH - does some ingredient in Boost pH cause the hanna alk color to vary from its normal ph-based color.
I've also heard one or two people say that Prime boosts their hanna alk result.
It just happens that I have a boat load of Hanna reagent and Prime left over from the little study we did. I have CloramX too.
- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
What is the dye in the Hanna? Maybe it gets chemically reduced by Prime.
The best that I can do is grab the Vis spectrum before and after Prime at constant pH.
- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
I was thinking of measuring the Vis spectrum and pH vs alkalinity to show how the method works. I assume the reagent is a mixture of acid and dye(s) and Hanna correlated color intensity to buffer capacity. Maybe I can find where non-linearity kicks in.I don't actually have a good grasp on how hanna alk works, beyond your general explanations of what it likely does.
- Joined
- May 22, 2016
- Messages
- 6,559
- Reaction score
- 10,130
there is only one piece of interesting info in the hanna SDS...I assume the reagent is a mixture of acid and dye(s) and Hanna correlated color intensity to buffer capacity.
So pH of 3.7, we can ponder what would happen to final pH if you mix a fixed amount of weak acid with a variable alkalinity saltwater.
the final pH might be something that you could map to the initial saltwater alkalinity.
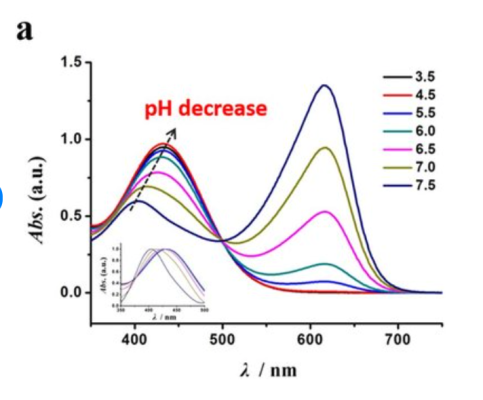
hanna says "The reaction causes a distinctive range of colors from yellow to green to blue to develop." So something like univesal indicator or bromothymol blue could give those colors in the likely pH ranges...
And if your checker LED is 610nm, then an indicator like that is well situated to quantify the pH from the absorbance.
Bromothymol blue....
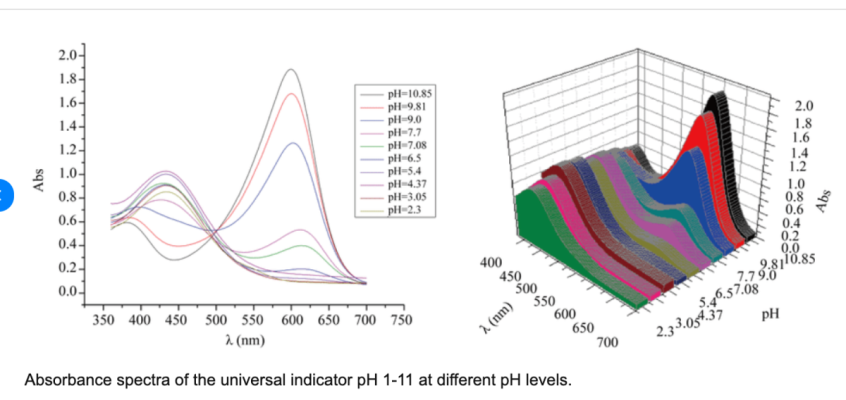
Universal indicator....
- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
Here is how the visible spectrum of the Hanna alkalinity test solution changes with increasing alkalinity.there is only one piece of interesting info in the hanna SDS...

So pH of 3.7, we can ponder what would happen to final pH if you mix a fixed amount of weak acid with a variable alkalinity saltwater.
the final pH might be something that you could map to the initial saltwater alkalinity.
hanna says "The reaction causes a distinctive range of colors from yellow to green to blue to develop." So something like univesal indicator or bromothymol blue could give those colors in the likely pH ranges...
And if your checker LED is 610nm, then an indicator like that is well situated to quantify the pH from the absorbance.
Bromothymol blue....

Universal indicator....

I titrated a solution of sodium bicarbonate to determine its concentration and then dosed it to 400 mL aquarium water. After each dose, I sampled the aquarium water and measured the alkalinity with the Checker, and then measured the pH and visible spectrum of the Checker solution. The hump on the left goes down while the right hand peak height increases. The Checker uses a 619 nm LED to measure light transmittance through the solution. I will have more to say about the Checker performance.
- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
Still waiting delivery of a Red Sea alkalinity test to wrap up the subject of testing alkalinity with a Hanna Checker. In the meantime, tested the concern that Boost pH+ behaves differently in Instant Ocean than it does in aquarium water because of the organic content. For my rank water and Instant Ocean there was no difference. Here’s the demonstration.
I removed a a sample of aquarium water and adjusted the alkalinity and pH to bring them close to that of Instant Ocean. I then added Boost pH+ (0.23 N) and measured the pH increase. Below is the comparison between tank water and Instant Ocean. The two curves are parallel over the entire range of Boost pH+ addition. If I had tweaked the aquarium water pH 0.05 units, the curves would have overlapped.
I feel we can add to the study conclusions that Boost pH+ behaves the same in both IO and tank water, and therefore, there is no organic chemical effect, positive or negative.
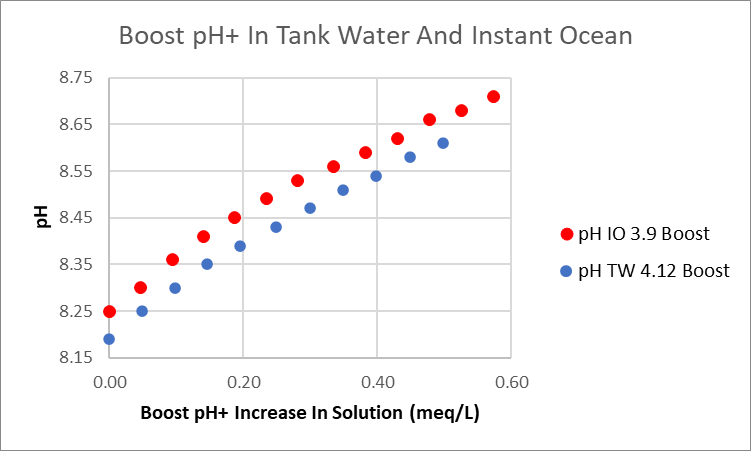
I removed a a sample of aquarium water and adjusted the alkalinity and pH to bring them close to that of Instant Ocean. I then added Boost pH+ (0.23 N) and measured the pH increase. Below is the comparison between tank water and Instant Ocean. The two curves are parallel over the entire range of Boost pH+ addition. If I had tweaked the aquarium water pH 0.05 units, the curves would have overlapped.
I feel we can add to the study conclusions that Boost pH+ behaves the same in both IO and tank water, and therefore, there is no organic chemical effect, positive or negative.
- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
Took a quick look at this with my Prime opened about a year ago. For reference, 2 drops, ~0.12 mL, in 4 L is the recommended dose.There's an aspect of what you are looking at that I think is really interesting.
I wonder if you make a solution of the hanna alk reagent in saltwater and look at the color vs pH - does some ingredient in Boost pH cause the hanna alk color to vary from its normal ph-based color.
I've also heard one or two people say that Prime boosts their hanna alk result.
A 33x recommended dose showed a 0.02 meq/L increase, i.e., no effect. A 222x recommended dose showed a 0.34 meq/L increase.
Maybe a fresher reagent and considerable overdosing might produce an observable effect. Could be interesting to look at the visible spectrum for the development of a new absorbance. I wonder what bleach or peroxide does to the dye.
Forgot to report that a total pH drop of 0.2 units occurred from a high of 8.25.
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,431
- Reaction score
- 63,793
Still waiting delivery of a Red Sea alkalinity test to wrap up the subject of testing alkalinity with a Hanna Checker. In the meantime, tested the concern that Boost pH+ behaves differently in Instant Ocean than it does in aquarium water because of the organic content. For my rank water and Instant Ocean there was no difference. Here’s the demonstration.
I removed a a sample of aquarium water and adjusted the alkalinity and pH to bring them close to that of Instant Ocean. I then added Boost pH+ (0.23 N) and measured the pH increase. Below is the comparison between tank water and Instant Ocean. The two curves are parallel over the entire range of Boost pH+ addition. If I had tweaked the aquarium water pH 0.05 units, the curves would have overlapped.
I feel we can add to the study conclusions that Boost pH+ behaves the same in both IO and tank water, and therefore, there is no organic chemical effect, positive or negative.

Great, thanks for the additional experiment.
Similar threads
- Replies
- 19
- Views
- 378
- Replies
- 8
- Views
- 260
New Posts
-
How much did you spend on your yellow tail tamarin wrasse?
- Latest: mrpizzaface
-


















