- Joined
- May 22, 2016
- Messages
- 6,600
- Reaction score
- 10,191
Here's Dan's comparison overlayed. It makes the text annoying, but the Graph axes are overlayed precisely which makes the comparison pretty clear.
The pH trends (orange data) are the same, and the measured alkalinity...
Blue circles for NaOH and Red circles for boost pH simply could not be any closer.
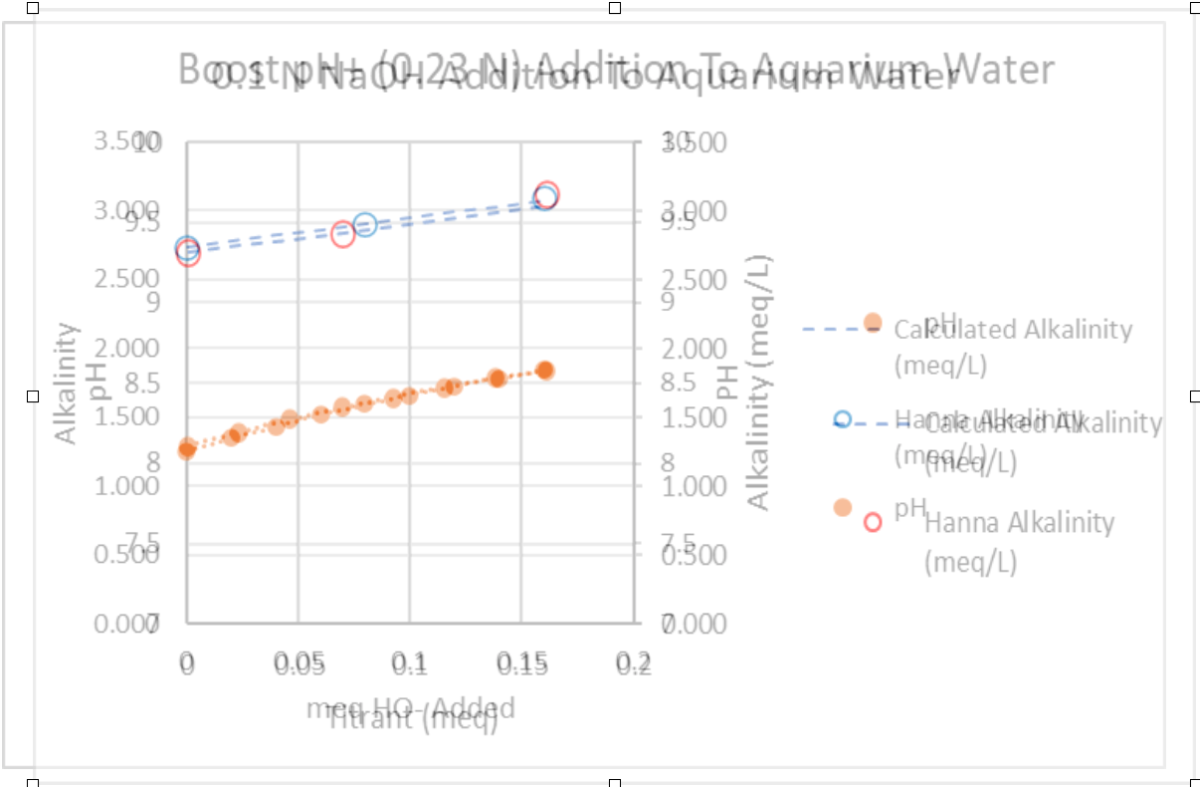
The pH trends (orange data) are the same, and the measured alkalinity...
Blue circles for NaOH and Red circles for boost pH simply could not be any closer.


















