If I raise my alkalinity around .5 dkh, will this have a measurable affect on my pH? If not, will 1 dkh? Is there a calculator for this type of equation?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
ffect of .5 Increase in Alkalinity on pH
- Thread starter chrisjj625
- Start date
- Tagged users None
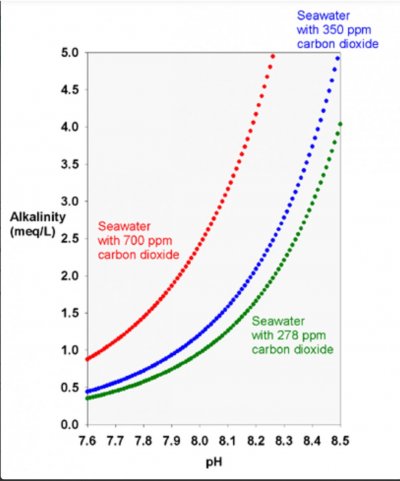
Assuming an Alk of 8.0dKH and C02 around 600 you would have a pH of 8.13. Increasing alk by 0.5 to 8.5dKH and no change in C02, your pH would increase to 8.15. So an increase of 0.02.
Now if you increase to 11dKH and no change to CO2, your pH would increase to 8.25.
Now if you increase to 11dKH and no change to CO2, your pH would increase to 8.25.
I'm assuming the question is not about the temp increase, but long term increase due to higher Alkalinity.Depends on what alk product you are using.
Yes long term. I struggle with low pH and have limited ability to do anything about it. So I was hoping a .5 -1 dkh increase might assist the problem.I'm assuming the question is not about the temp increase, but long term increase due to higher Alkalinity.
Thank you! Is there anything like this with dkh since that is what I am measuring. Converting it all is a PIA, but I can do it, if nothing better exists.Courtesy of a Randy Holmes Farley article;
Thank you! Is there anything like this with dkh since that is what I am measuring. Converting it all is a PIA, but I can do it, if nothing better exists.
Alkalinity Conversion Calculator
Aquarium calculator; Convert alkalinity between milliequivalents-per-liter (meq/L, meql), carbonate hardness (dKH, KH), and parts-per-million (ppm).
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,509
- Reaction score
- 63,933
If I raise my alkalinity around .5 dkh, will this have a measurable affect on my pH? If not, will 1 dkh? Is there a calculator for this type of equation?
You may mean one of two entirely different questions,
1. If I boost alk with product X by 1 dKH, how much does alk rise or fall instantly?
2. If my tank has an alk higher by 1 dKH, how much higher will my pH be, everything else identical?
Which are you asking?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,509
- Reaction score
- 63,933
Thank you! Is there anything like this with dkh since that is what I am measuring. Converting it all is a PIA, but I can do it, if nothing better exists.
Nothing better exists than my articles, all of them. lol
2You may mean one of two entirely different questions,
1. If I boost alk with product X by 1 dKH, how much does alk rise or fall instantly?
2. If my tank has an alk higher by 1 dKH, how much higher will my pH be, everything else identical?
Which are you asking?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,509
- Reaction score
- 63,933
In that case, a rise of 0.5 dKH is associated with a rise in pH of about 0.02 to 0.03 pH units, depending on the starting alk.
Thank you! I was hoping for a higher number, but I appreciate the info!In that case, a rise of 0.5 dKH is associated with a rise in pH of about 0.02 to 0.03 pH units, depending on the starting alk.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,509
- Reaction score
- 63,933
Thank you! I was hoping for a higher number, but I appreciate the info!
Yes, that's why I do not generally recommend electing an alk based on a desired to attain a different pH.
Similar threads
- Replies
- 1
- Views
- 71
- Replies
- 21
- Views
- 444
- Replies
- 27
- Views
- 413
- Replies
- 7
- Views
- 306
- Replies
- 20
- Views
- 445