Ok Randy or whoever else can help here, got a good one for you guys.
Primary Goal: Use less DI Media
Secondary Goal: Maybe have cleaner/better drinking water
Ok guys let's start with this:
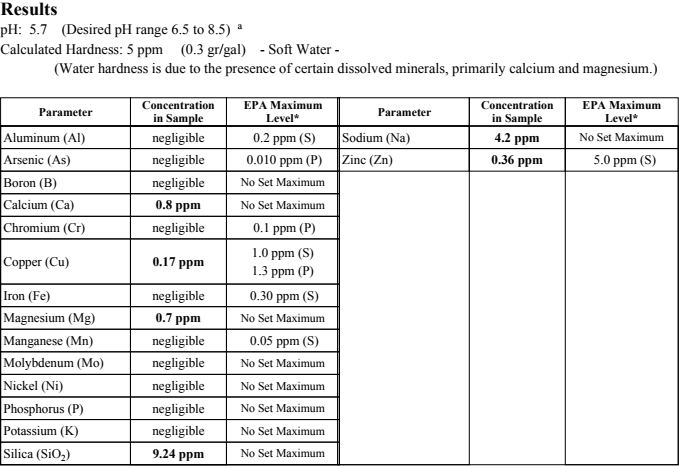
As can be seen there's basically nothing in my water but water and silica. This test was taken from the kitchen sink and my RODI system hooks into the washing machine inlet which is all PVC from the well to the washer so I know I can eliminate at minimum the copper.
It's my understanding there are two ways to to raise water pH. I can aerate the water or I can use a whole house Acid Neutralizer. I'm currently considering both options.
Obviously a degas unit for the whole house would be too expensive so if I go the degas route it would have to be done locally at the RODI unit which I can do.
My other option becomes the Acid Neutralizer which uses Calcite and Corosex. Calcite is Calcium Carbonate which would obviously make my Calcium levels go up from .8ppm. Corosex is Magnesium Oxide.
Based on my pH I should be using a 90/10 blend of Calcium Carbonate/Magnesium Oxide.
What I'm trying to figure out and the reason for this thread is, how much strain would be put on my entire filtering system before the DI filter to remove the increased amounts of Calcium and Magneseum. I'm assuming Calcium and Magnesium is taken out of the water prior to the DI stage?
My planned system would look like this:
Sediment Filter > Neutralizer > Sediment Filter > Well Pump > Well Booster Pump (I already have this instelled) > RODI Unit
RODI configuration as follows:
5Micron Sediment> 1 Micron Sediment> 1Micron Carbon > Dual RO Membranes > DI Canister
Primary Goal: Use less DI Media
Secondary Goal: Maybe have cleaner/better drinking water
Ok guys let's start with this:

As can be seen there's basically nothing in my water but water and silica. This test was taken from the kitchen sink and my RODI system hooks into the washing machine inlet which is all PVC from the well to the washer so I know I can eliminate at minimum the copper.
It's my understanding there are two ways to to raise water pH. I can aerate the water or I can use a whole house Acid Neutralizer. I'm currently considering both options.
Obviously a degas unit for the whole house would be too expensive so if I go the degas route it would have to be done locally at the RODI unit which I can do.
My other option becomes the Acid Neutralizer which uses Calcite and Corosex. Calcite is Calcium Carbonate which would obviously make my Calcium levels go up from .8ppm. Corosex is Magnesium Oxide.
Based on my pH I should be using a 90/10 blend of Calcium Carbonate/Magnesium Oxide.
What I'm trying to figure out and the reason for this thread is, how much strain would be put on my entire filtering system before the DI filter to remove the increased amounts of Calcium and Magneseum. I'm assuming Calcium and Magnesium is taken out of the water prior to the DI stage?
My planned system would look like this:
Sediment Filter > Neutralizer > Sediment Filter > Well Pump > Well Booster Pump (I already have this instelled) > RODI Unit
RODI configuration as follows:
5Micron Sediment> 1 Micron Sediment> 1Micron Carbon > Dual RO Membranes > DI Canister
















