- Joined
- Sep 20, 2018
- Messages
- 1,117
- Reaction score
- 1,090
Link some of those studies then.
I run a calcium reactor. The effluent is about pH 6.2. I can leave a cup of it out, and it's at almost 8 in less than an hour. I know, because I've done this.

A new experimental technique for validating exchange models of carbon dioxide between the atmosphere and sea-water - PubMed
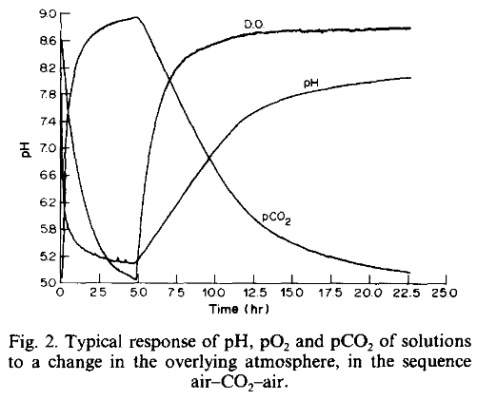
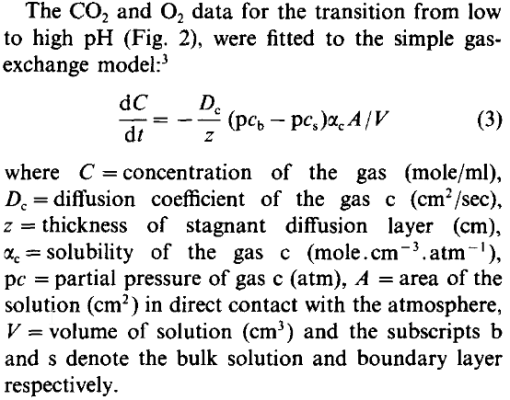
The mechanism of CO(2)-exchange between the atmosphere and sea-water was re-examined by simultaneously measuring pH and pO(2) in artificial sea-water exposed to CO(2) and air atmospheres. The data were fitted to an exchange model by using both the differential and integral forms of the diffusion...
CO2 is much more soluble than O2 but their coefficients of diffusion are similar. My tap water comes out at 120% O2 (I don't have a CO2 meter) saturation and even with heating and a mixing pump it takes more than 24 hours to reach equilibrium.
Fish farms have degassing towers to remove CO2. If CO2 instantly diffused out of water there would be no need.
Gases don't diffuse out of water instantly or even rapidly in most cases.
Someone on theplantedtank.net tested pH of a bag of shipped fish and said it didn't obey the narrative. That's a better claim than yours due to context alone.
Attachments
-
 2020-04-09 01_19_16-Sci-Hub _ A new experimental technique for validating exchange models of c...png30.4 KB · Views: 29
2020-04-09 01_19_16-Sci-Hub _ A new experimental technique for validating exchange models of c...png30.4 KB · Views: 29 -
 2020-04-09 01_19_46-Sci-Hub _ A new experimental technique for validating exchange models of c...png75.9 KB · Views: 37
2020-04-09 01_19_46-Sci-Hub _ A new experimental technique for validating exchange models of c...png75.9 KB · Views: 37 -
 2020-04-10 14_48_19-Sci-Hub _ A new experimental technique for validating exchange models of c...png53.7 KB · Views: 38
2020-04-10 14_48_19-Sci-Hub _ A new experimental technique for validating exchange models of c...png53.7 KB · Views: 38



















