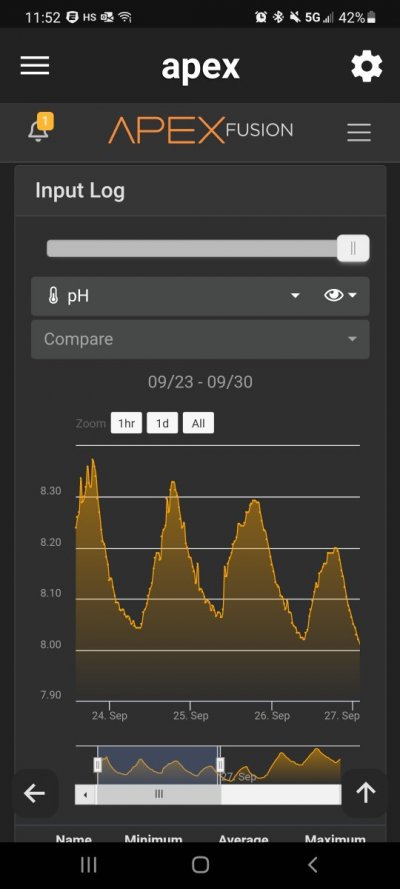
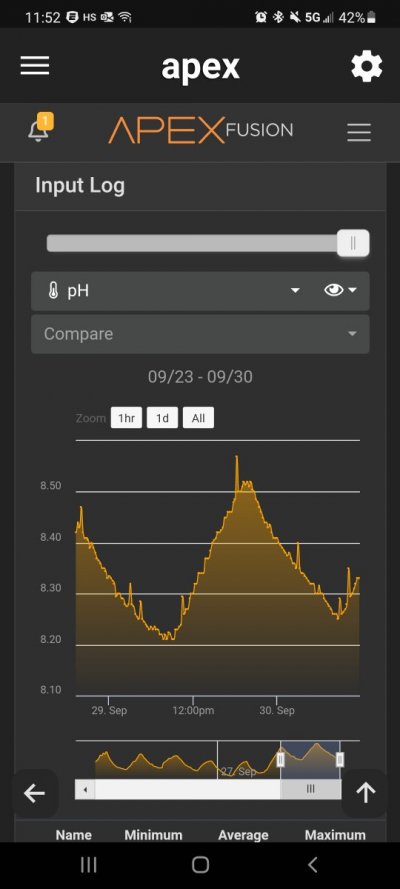
Post in thread 'Aquarium pH Distilled' https://www.reef2reef.com/threads/aquarium-ph-distilled.853474/post-9330359
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
If you pH is less than 7.8, your pH meter is probably wrong
- Thread starter arking_mark
- Start date
- Tagged users None
Bleigh
The best bad influence
View Badges
Reef Squad
Partner Member 2024
Excellence Award
Reef Tank 365
Hospitality Award
RASOC Member
CRC Member
My Tank Thread
Every ph meter I have reads a different number, anywhere from 8-8.6. SOOOO important to calibrate them regularly.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,276
- Reaction score
- 63,629
I don't believe the title statement is true. 
For me low PH means not enough oxygen in your water.
Bleigh
The best bad influence
View Badges
Reef Squad
Partner Member 2024
Excellence Award
Reef Tank 365
Hospitality Award
RASOC Member
CRC Member
My Tank Thread
For me low PH means not enough oxygen in your water.
The original post said "assuming good aeration".
@arking_mark Can you copy and paste the chart you linked to? I assume this is a separate conversation from your original post.
Interesting stuff!
PeterC99
Solarbenchmark.com
View Badges
Excellence Award
Reef Tank 365
NJRC Member
Hudson Valley Reef Keepers
Hospitality Award
My Tank Thread
My Aquarium Showcase
Aren’t there other factors that could cause pH below 7.8?
For example:
Your saltwater mix
Lack of sufficient gas exchange
Nitric acid build-up from biological filtration
Production of organic acids from metabolic waste products.
For example:
Your saltwater mix
Lack of sufficient gas exchange
Nitric acid build-up from biological filtration
Production of organic acids from metabolic waste products.
Bleigh
The best bad influence
View Badges
Reef Squad
Partner Member 2024
Excellence Award
Reef Tank 365
Hospitality Award
RASOC Member
CRC Member
My Tank Thread
Aren’t there other factors that could cause pH below 7.8?
For example:
Your saltwater mix
Lack of sufficient gas exchange
Nitric acid build-up from biological filtration
Production of organic acids from metabolic waste products.
I suspect the OP intended to have conversation on inaccuracy of testing equipment based on the link that was posted. Some of those things were discussed in that thread... including being able to tell when an unexpected person arrived at the house based on PH levels.

Aren’t there other factors that could cause pH below 7.8?
For example:
Your saltwater mix
Lack of sufficient gas exchange
Nitric acid build-up from biological filtration
Production of organic acids from metabolic waste products.
A bad batch of salt - maybe, but the Alk content would have to be super low
Lack of sufficient gas exchange - yes, covered in the link
The nitrogen cycle will consume Alk as NO3 builds up in the tank but release it back as it NO3 get broken down later in the cycle. So unless it tanks the Alk...I don't think this would cause the tank to go below 7.8
I'm not knowledgeable on organic acid production from metabolic waste products. I'll defer to @Randy Holmes-Farley. However, I'm not sure this would be any different than the nitrogen cycle...
Lol. pH is not a measure of Oxygen.For me low PH means not enough oxygen in your water.
I don't believe the title statement is true.
Why? Happy to learn and modify or remove it.
Why? Happy to learn and modify or remove it.
From the link...
If your pH is less than 7.8, it can be from the following causes:
- Inadequate tank aeration that leads to the accumulation of CO2
- pH-lowering dosing such as a calcium carbonate reactor
- Super-high stocking levels that would cause in-tank issues
- Unhealthy levels of CO2 in your tank location or house that would cause you noticeable health issues
So if your tank has good aeration, your house or tank area has normal indoor levels of CO2, your tank is not super overstocked, and you are not dosing anything that lowers pH, any reading below 7.8 is not accurate. Your meter is not giving a realistic number.
Bleigh
The best bad influence
View Badges
Reef Squad
Partner Member 2024
Excellence Award
Reef Tank 365
Hospitality Award
RASOC Member
CRC Member
My Tank Thread
From the link...
If your pH is less than 7.8, it can be from the following causes:
#3 & #4 are super unlikely as you would notice these issues. #4 would cause you health issues such as headaches, lethargy, etc... These are super easy to rule out and super uncommon.
- Inadequate tank aeration that leads to the accumulation of CO2
- pH-lowering dosing such as a calcium carbonate reactor
- Super-high stocking levels that would cause in-tank issues
- Unhealthy levels of CO2 in your tank location or house that would cause you noticeable health issues
So if your tank has good aeration, your house or tank area has normal indoor levels of CO2, your tank is not super overstocked, and you are not dosing anything that lowers pH, any reading below 7.8 is not accurate. Your meter is not giving a realistic number.
I just don't think people followed the link to know that this was a side trail from the original post.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,276
- Reaction score
- 63,629
Why? Happy to learn and modify or remove it.
Why would it need to be a mismeasurement?
Tanks often have excess CO2 relative to the air at night.
Even if one assumed equilibrium at the nightly low, pH 7.7 at 7 dKH alk means about 1250 ppm CO2 in the air.
That's hardly unusual:
High levels of carbon dioxide in house - GreenBuildingAdvisor
My house has high levels of CO2 / carbon dioxide, every room is between 1100 ppm to 1200 ppm according to an air quality test I had. There are only 2 grown occupants and it's a 1000 sq. ft. brick house. No pets or plants, gas stove / furnace / water heater. The basement was the only area that...
"My house has high levels of CO2 / carbon dioxide, every room is between 1100 ppm to 1200 ppm according to an air quality test I had. There are only 2 grown occupants and it’s a 1000 sq. ft. brick house. No pets or plants, gas stove / furnace / water heater."
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,276
- Reaction score
- 63,629
I just don't think people followed the link to know that this was a side trail from the original post.
Regardless, since the title is wrong, I do not think it appropriate to just follow the link and leave a flashing light of misinformation.
Bleigh
The best bad influence
View Badges
Reef Squad
Partner Member 2024
Excellence Award
Reef Tank 365
Hospitality Award
RASOC Member
CRC Member
My Tank Thread
Regardless, since the title is wrong, I do not think it appropriate to just follow the link and leave a flashing light of misinformation.
That's why I suggested that he edit the first post to include the information instead of just linking to it. I think a conversation about when to trust your testing equipment and how to know you need to calibrate is a good conversation to have. More knowledge, more power.

Why would it need to be a mismeasurement?
Tanks often have excess CO2 relative to the air at night.
Even if one assumed equilibrium at the nightly low, pH 7.7 at 7 dKH alk means about 1250 ppm CO2 in the air.
That's hardly unusual:
High levels of carbon dioxide in house - GreenBuildingAdvisor
My house has high levels of CO2 / carbon dioxide, every room is between 1100 ppm to 1200 ppm according to an air quality test I had. There are only 2 grown occupants and it's a 1000 sq. ft. brick house. No pets or plants, gas stove / furnace / water heater. The basement was the only area that...www.greenbuildingadvisor.com
"My house has high levels of CO2 / carbon dioxide, every room is between 1100 ppm to 1200 ppm according to an air quality test I had. There are only 2 grown occupants and it’s a 1000 sq. ft. brick house. No pets or plants, gas stove / furnace / water heater."
i can confirm 1200 isn’t a crazy amount of co2, I bought a decent meter and we were hitting that especially with the AC in summer on with just 2 adults. I had an HRV installed and that dropped co2 to around 800 max I have seen. If one has high co2 and using a calcium reactor as well pH can get pretty dang low.
Why would it need to be a mismeasurement?
Tanks often have excess CO2 relative to the air at night.
Even if one assumed equilibrium at the nightly low, pH 7.7 at 7 dKH alk means about 1250 ppm CO2 in the air.
That's hardly unusual:
High levels of carbon dioxide in house - GreenBuildingAdvisor
My house has high levels of CO2 / carbon dioxide, every room is between 1100 ppm to 1200 ppm according to an air quality test I had. There are only 2 grown occupants and it's a 1000 sq. ft. brick house. No pets or plants, gas stove / furnace / water heater. The basement was the only area that...www.greenbuildingadvisor.com
"My house has high levels of CO2 / carbon dioxide, every room is between 1100 ppm to 1200 ppm according to an air quality test I had. There are only 2 grown occupants and it’s a 1000 sq. ft. brick house. No pets or plants, gas stove / furnace / water heater."
I still get a pH of 7.8 NBS with 7 Alk and 1250 ppm CO2 level. Again assuming a well aerated tank.
A friend just changed out his sump on his tank. His original had multiple baffles. His new one has only one. His PH is now 7.8 where in the past it was 8.3Lol. pH is not a measure of Oxygen.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,276
- Reaction score
- 63,629
A friend just changed out his sump on his tank. His original had multiple baffles. His new one has only one. His PH is now 7.8 where in the past it was 8.3
I'm not sure how that is a response to the fact that pH and O2 are not directly related.
CO2 is the gas that controls pH.
CO2 is sometimes is related to O2 (say, by photosynthesis or respiration) and sometimes not (say, by alk additives consuming CO2, or CaCO3/CO3 reactor adding CO2, etc.).
Changing any mechanical aquarium aspect that alters aeration may impact O2, but the effect on pH is by altering CO2 exchange. Your friend is probably driving high CO2 air into the water with those baffles.
Similar threads
- Replies
- 8
- Views
- 187
- Replies
- 2
- Views
- 78
- Replies
- 6
- Views
- 577
- Replies
- 399
- Views
- 20,721
New Posts
-
Build Thread 32 EXT Fiji Cube - SPS Mini Haven - Nano Dream Build
- Latest: JustAnotherNanoTank
-