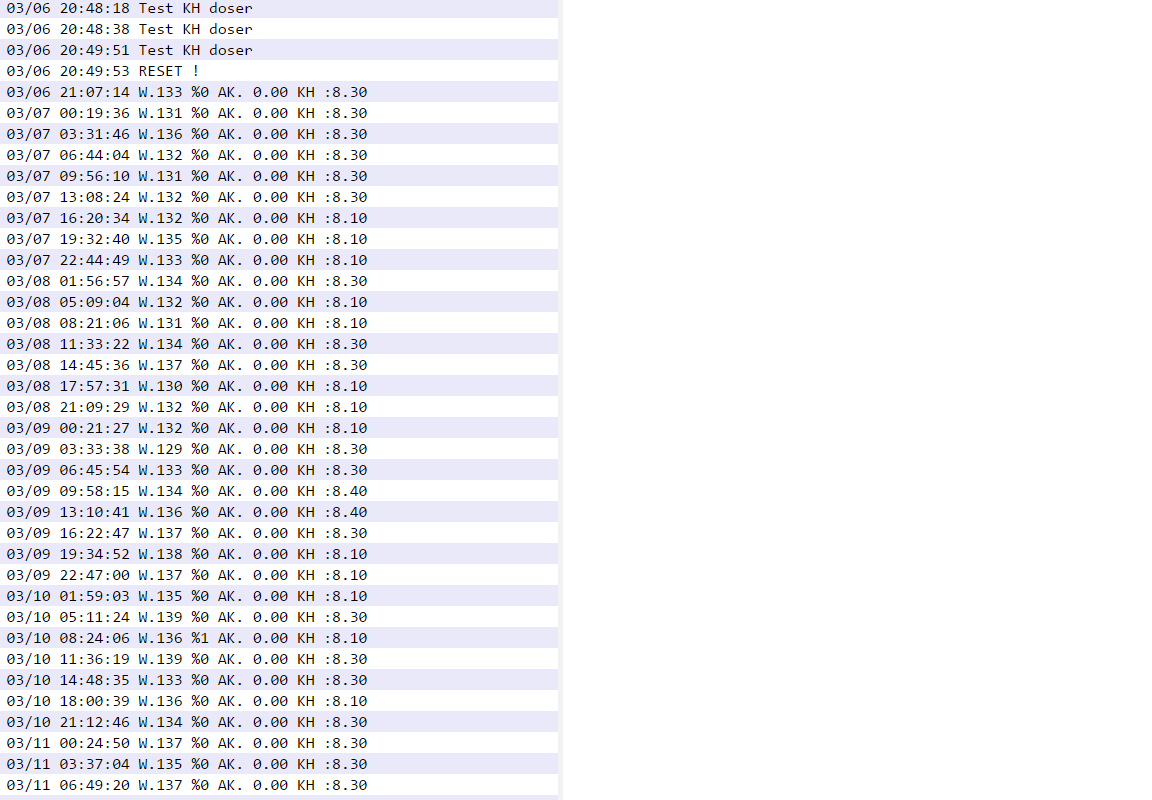
Below is my current view comparing KH Guardian to my daily Alk testing w/ Redsea.
1. I care about repeatability and ability to watch trends and not the actual number. As long as the unit is within .5 DKH accuracy I am ok, I care about repeatabilty. Missing numbers make it tough and I am still waiting/letting my system drop to a location w/ no missing #.
2. When I test with my Redsea test kit, I never react to a a single day fluctuation so I watch general patterns over time and mentally see if the "average" goes down or up. Also, I personally always record a tritation range, and not a value (eg. 7.6-.7.7). To me, the KH "jumping" values is the same thing. As long as I watch the pattern....
3. To me, the KH Guardian provides the same level of benefit in terms of monitoring as me testing once a day every day as long as I focus on overall pattern. Except I am not doing it every day myself!
4. With that said, I currently feel I can stop daily testing and watch the KH pattern and ignore any "noise" or jump in value and effectively ignore it unless its repeated between tests and indicates a trend. No reason to test more than every few hours....
I currently have my KH target set to 8.1, and lowered my alkalinity to around 8.1 and leveled off my reactor (DaStaCo). I think I leveled it too good since I haven't had a drop below 8.1! I will leave it here and let my consumption slowly rise and the KH to "catch it" when it does. That may take some time but I won't rush it.

1. I care about repeatability and ability to watch trends and not the actual number. As long as the unit is within .5 DKH accuracy I am ok, I care about repeatabilty. Missing numbers make it tough and I am still waiting/letting my system drop to a location w/ no missing #.
2. When I test with my Redsea test kit, I never react to a a single day fluctuation so I watch general patterns over time and mentally see if the "average" goes down or up. Also, I personally always record a tritation range, and not a value (eg. 7.6-.7.7). To me, the KH "jumping" values is the same thing. As long as I watch the pattern....
3. To me, the KH Guardian provides the same level of benefit in terms of monitoring as me testing once a day every day as long as I focus on overall pattern. Except I am not doing it every day myself!
4. With that said, I currently feel I can stop daily testing and watch the KH pattern and ignore any "noise" or jump in value and effectively ignore it unless its repeated between tests and indicates a trend. No reason to test more than every few hours....
I currently have my KH target set to 8.1, and lowered my alkalinity to around 8.1 and leveled off my reactor (DaStaCo). I think I leveled it too good since I haven't had a drop below 8.1! I will leave it here and let my consumption slowly rise and the KH to "catch it" when it does. That may take some time but I won't rush it.





















