I am down to my last box of Salifert Alkalinity while breaking in a new Calcium reactor. So I needed to dilute my sample to avoid wasting too much of the second reagent.
Effluent dkh calculation -- can someone check me out on this Salifert calculation?
a) 1 part effluent to 4 parts RODI
b) Four drops of reagent #1
c) 1.3 ml of reagent #2 to get the color change
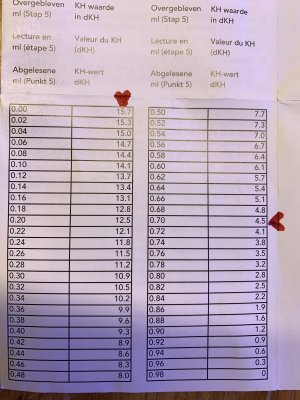
d) Therefore, 15.7 + 4.5 = 20.2 diluted
e) 20.2 X 4 = 80.8 dkh undiluted
Am I thinking about this the right way?
This picture will help you figure out where I got the 15.7 and 4.5 numbers from.
Effluent dkh calculation -- can someone check me out on this Salifert calculation?
a) 1 part effluent to 4 parts RODI
b) Four drops of reagent #1
c) 1.3 ml of reagent #2 to get the color change
d) Therefore, 15.7 + 4.5 = 20.2 diluted
e) 20.2 X 4 = 80.8 dkh undiluted
Am I thinking about this the right way?
This picture will help you figure out where I got the 15.7 and 4.5 numbers from.



















