- Joined
- May 6, 2020
- Messages
- 222
- Reaction score
- 92
From the post below, and other posts of his, I think that @Randy Holmes-Farley believes that being very concerned about hydrogen sulfide and sulfur denitrators is not justifiable. I agree with him if that's what he believes.
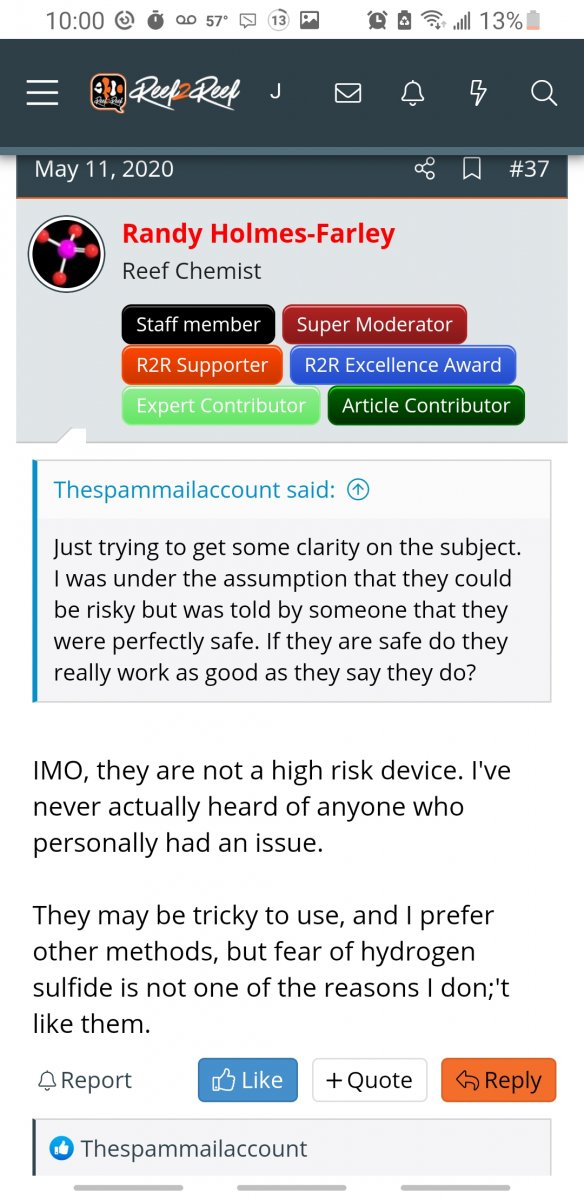
The thread is called "can a sulfur denitrator be dangerous?"
The thread is called "can a sulfur denitrator be dangerous?"
Last edited:


















