Hello,
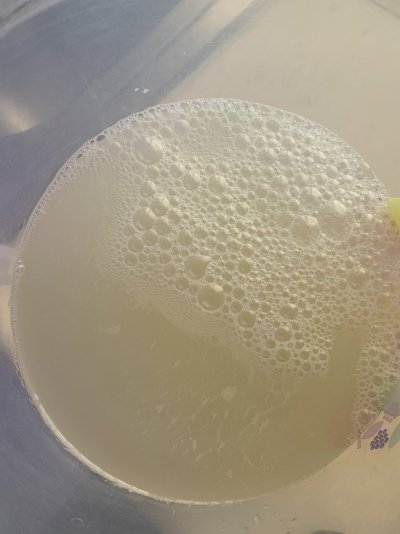
I’ve put 594g of sodium bicarbonate in the oven for 1 hour to make sodium carbonate but I’m trying to mix it and after a few hours the solution does not clear up. It has a brown-yellow color and I’m not able to see the bottom of the 1 gallon jug.
Any reason? Is this normal?
I’ve put 594g of sodium bicarbonate in the oven for 1 hour to make sodium carbonate but I’m trying to mix it and after a few hours the solution does not clear up. It has a brown-yellow color and I’m not able to see the bottom of the 1 gallon jug.
Any reason? Is this normal?