- Joined
- Sep 21, 2018
- Messages
- 6,773
- Reaction score
- 7,248
Two recent posts
https://www.reef2reef.com/threads/analyzing-a-bacterial-method-for-dinoflagellates-and-cyano.635165/
https://www.reef2reef.com/threads/what-does-adding-hydrogen-peroxide-do-to-tank-chemistry-and-organizims-in-tank.640936/
contained information about the use of hydrogen peroxide that made me wonder whether hydrogen peroxide actually worked as described. Both the claim of what hydrogen peroxide does and the seemingly small effective concentration at which it achieves these things raised several questions, the first being “does it even survive in the aquarium.” This post provides data that we might use to answer the question, at least provisionally.
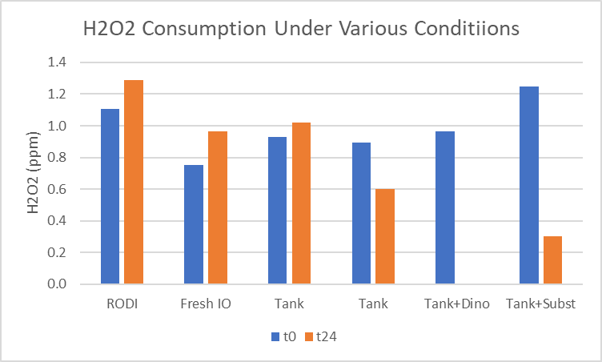
Hydrogen peroxide (3%) was added to a range of solutions and the resulting ~1 ppm solutions held at room temperature for twenty fours. Using the Hanna hydrogen peroxide test, the concentration of hydrogen peroxide was determined at the beginning and end of the hold period (bar chart). As expected, hydrogen peroxide was stable in RO/DI. The variation in the height of the first pair of bars gives an indication of the variation of the method in my hands.
Hydrogen peroxide mixed with freshly prepared Instant Ocean and two samples of aquarium water also showed little or no hydrogen peroxide degradation. When dinoflagellates and substrate were mixed with aquarium water, most or all of the hydrogen peroxide was consumed (last two pairs of bars). This suggests hydrogen peroxide could be totally consumed when added to an aquarium? How long would 1 ppm of hydrogen peroxide last in an aquarium?
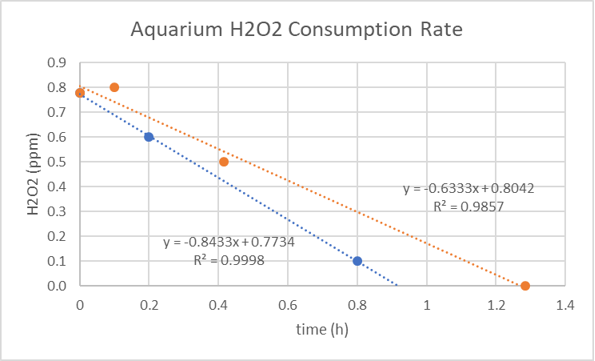
The second graph shows the results of two consecutive doses to my fish only aquarium. 1 ppm is consumed in about one hour. Does this happen in every aquarium? Does the rate vary by time of day? What things are responsible for hydrogen peroxide consumption? Is the consumption a “useful consumption”, e.g., killing or incapacitating a microorganism, or just the conversion of H2O2 to water and O2?
I had an offline discussion with @brandon429 on the sort of experiments that might be useful concerning hydrogen peroxide dosing. Maybe this data set has raised some questions you would like answered. Let me know.
https://www.reef2reef.com/threads/analyzing-a-bacterial-method-for-dinoflagellates-and-cyano.635165/
https://www.reef2reef.com/threads/what-does-adding-hydrogen-peroxide-do-to-tank-chemistry-and-organizims-in-tank.640936/
contained information about the use of hydrogen peroxide that made me wonder whether hydrogen peroxide actually worked as described. Both the claim of what hydrogen peroxide does and the seemingly small effective concentration at which it achieves these things raised several questions, the first being “does it even survive in the aquarium.” This post provides data that we might use to answer the question, at least provisionally.
Hydrogen peroxide (3%) was added to a range of solutions and the resulting ~1 ppm solutions held at room temperature for twenty fours. Using the Hanna hydrogen peroxide test, the concentration of hydrogen peroxide was determined at the beginning and end of the hold period (bar chart). As expected, hydrogen peroxide was stable in RO/DI. The variation in the height of the first pair of bars gives an indication of the variation of the method in my hands.
Hydrogen peroxide mixed with freshly prepared Instant Ocean and two samples of aquarium water also showed little or no hydrogen peroxide degradation. When dinoflagellates and substrate were mixed with aquarium water, most or all of the hydrogen peroxide was consumed (last two pairs of bars). This suggests hydrogen peroxide could be totally consumed when added to an aquarium? How long would 1 ppm of hydrogen peroxide last in an aquarium?
The second graph shows the results of two consecutive doses to my fish only aquarium. 1 ppm is consumed in about one hour. Does this happen in every aquarium? Does the rate vary by time of day? What things are responsible for hydrogen peroxide consumption? Is the consumption a “useful consumption”, e.g., killing or incapacitating a microorganism, or just the conversion of H2O2 to water and O2?
I had an offline discussion with @brandon429 on the sort of experiments that might be useful concerning hydrogen peroxide dosing. Maybe this data set has raised some questions you would like answered. Let me know.



















