- Joined
- May 22, 2016
- Messages
- 6,559
- Reaction score
- 10,131
My tank is in a classroom, and I realized this means that the CO2 in the room may go quite high and this might make for an interesting demonstration of how the CO2 affects the tank pH. So here we go.
This is what the pH swing looks like when the skimmer intake line is run out the window.
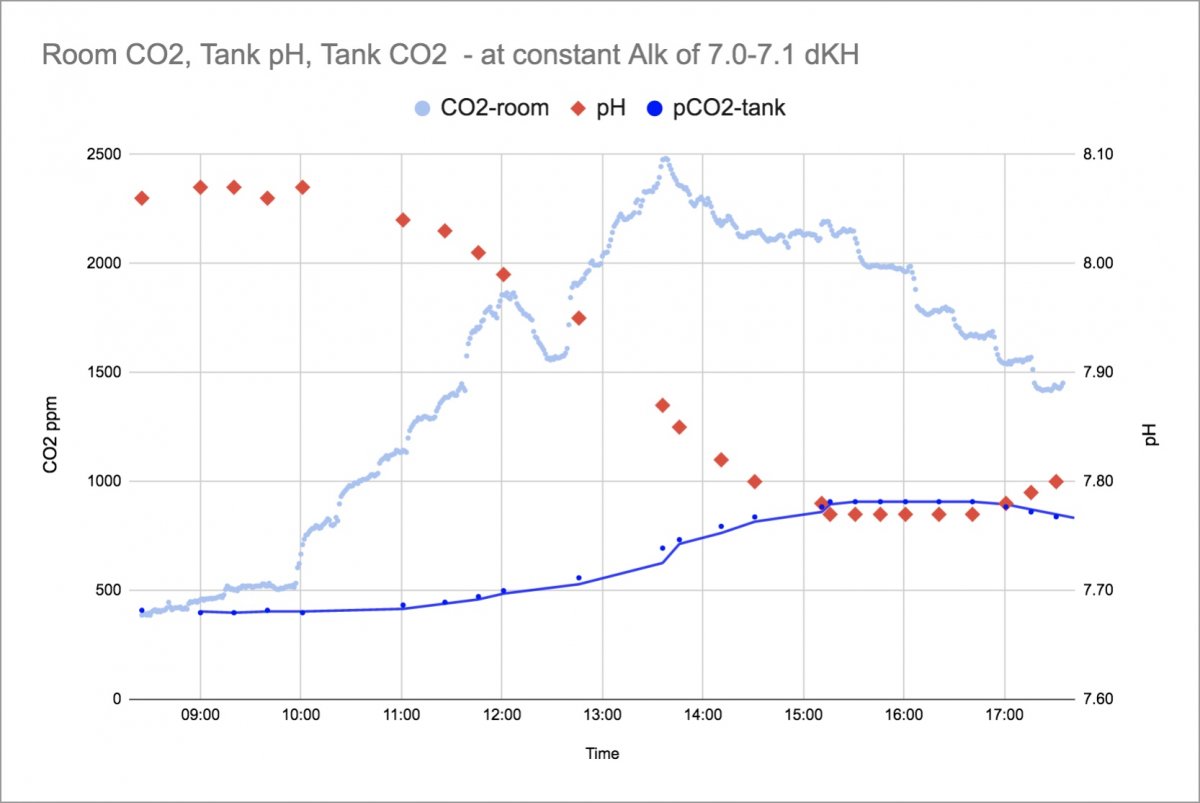
The Light Blue is the measured indoor CO2 of the room. It starts around 400ppm after the building has been empty for a weekend and climbs throughout the day (jumps, dips and stairsteps are due to movement of student groups of different sizes and A/C cutting on and off.) The indoor CO2 level peaks around 2500ppm. The Red data points are the pH measurements that showed a dip throughout the daytime from 8.07 down to 7.76 before slowly recovering. The Dark Blue is a calculated concentration of how much CO2 is in the water based on pH and Alk measurements. The Alk was held constant at 7.0 to 7.1 for this data.
A few more comments:
The calculated water CO2 starts the day in equilibrium with the inside/outside CO2 level, which is a nice check on the calculation method.
The downward effect on pH from this much CO2 is sizable, especially at this low alkalinity - even with the skimmer pulling in air from outside. It is not until the room CO2 is well down from its highs that the pH can start to recover. Pulling outside air seems to have helped quite a bit, as the tank water CO2 levels went from 400 to a max of only 900ppm. If the skimmer were pulling the indoor CO2 rich air at 2500ppm, the pH shift should be even more dramatic. Hopefully I'll get data on that for comparison soon.
This is what the pH swing looks like when the skimmer intake line is run out the window.
The Light Blue is the measured indoor CO2 of the room. It starts around 400ppm after the building has been empty for a weekend and climbs throughout the day (jumps, dips and stairsteps are due to movement of student groups of different sizes and A/C cutting on and off.) The indoor CO2 level peaks around 2500ppm. The Red data points are the pH measurements that showed a dip throughout the daytime from 8.07 down to 7.76 before slowly recovering. The Dark Blue is a calculated concentration of how much CO2 is in the water based on pH and Alk measurements. The Alk was held constant at 7.0 to 7.1 for this data.
A few more comments:
The calculated water CO2 starts the day in equilibrium with the inside/outside CO2 level, which is a nice check on the calculation method.
The downward effect on pH from this much CO2 is sizable, especially at this low alkalinity - even with the skimmer pulling in air from outside. It is not until the room CO2 is well down from its highs that the pH can start to recover. Pulling outside air seems to have helped quite a bit, as the tank water CO2 levels went from 400 to a max of only 900ppm. If the skimmer were pulling the indoor CO2 rich air at 2500ppm, the pH shift should be even more dramatic. Hopefully I'll get data on that for comparison soon.