Many people both in tropical/ subtropical and temperate zones have problem with the pH of the aquarium caused of excess CO2 in the house/apartment. Heat and cold (depending on climate zon) do not allow open windows, hence the CO2 concentration rise indoors. Even if it is possible to let outdoor air dominate even indoors - the outdoor air concentration of CO2 is around 420 ppm - it means that the equilibrium pH in the water is only around 8.1 - 8.15 - and outdoor CO2 is raising all the time.
The solution of this have been to scrub the Skimmers incoming air with a compound that was developed to take away CO2 in rebreathing techniques. It is basically Calcium dioxide. It works well but it is a costly process and you normally do not use the full capacity of the Calcium dioxide. Recirculation with humid air has been used in order to increase the lifetime of the calcium dioxide.
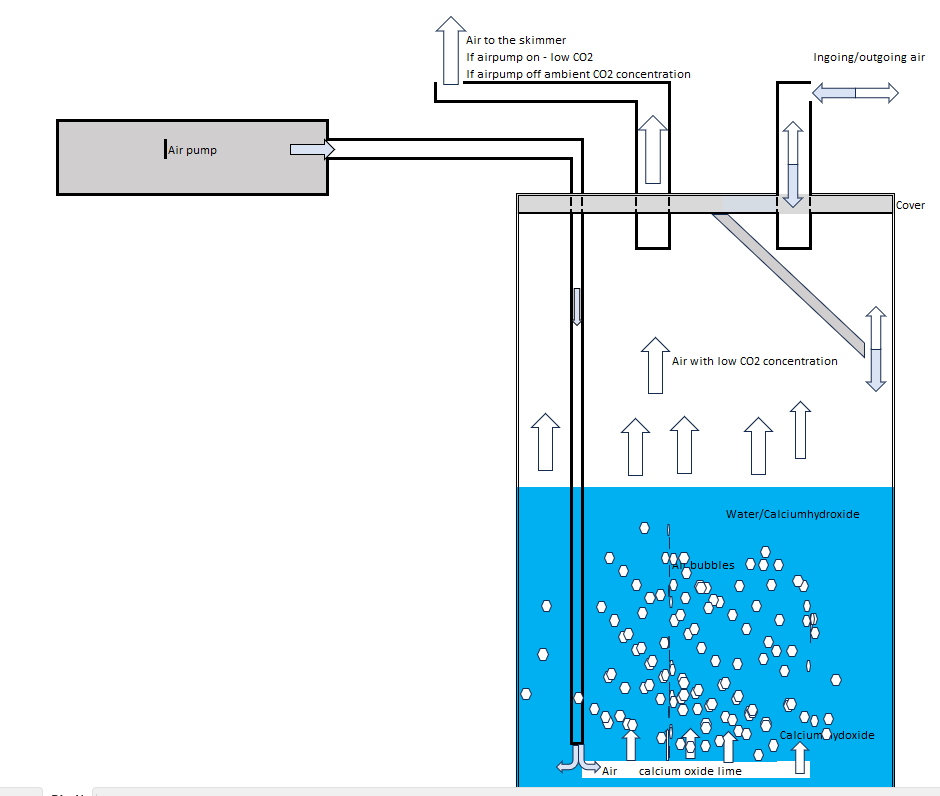
The optimal use of Calcium oxide and Calcium dioxide in order to catch CO2 would be to use a slurry. My idea is to use an normal air pump with an hourly air volume that is higher than the skimmer use. This pump will pump in air to the bottom of a jar containing Calcium oxide/ dioxide - of the type used in Kalk stirrer - and water. The concentration of Calcium oxide/dioxide - far more than saturation point. The air from the pump mix water, calcium oxid/hydroxid and air in a slurry. CO2 in the air will be transferred over to because of the high pH and The transferred CO2 will form CaCO3 (limestone). The air above the slurry will have low to zero concentrations of CO2. In the top of the jar - the skimmer sucks in the rather CO2 free air. The jar need also to have en opening out to the external environment - normally to let surplus air from the air pump let out but also to let air with CO2 go into the skimmer if the air pump stops. See figure
This is only an idea - have not tested it yet.
But is it a good idea worth to test or just one of those which should go directly into the trash?
Sincerely Lasse
The solution of this have been to scrub the Skimmers incoming air with a compound that was developed to take away CO2 in rebreathing techniques. It is basically Calcium dioxide. It works well but it is a costly process and you normally do not use the full capacity of the Calcium dioxide. Recirculation with humid air has been used in order to increase the lifetime of the calcium dioxide.
The optimal use of Calcium oxide and Calcium dioxide in order to catch CO2 would be to use a slurry. My idea is to use an normal air pump with an hourly air volume that is higher than the skimmer use. This pump will pump in air to the bottom of a jar containing Calcium oxide/ dioxide - of the type used in Kalk stirrer - and water. The concentration of Calcium oxide/dioxide - far more than saturation point. The air from the pump mix water, calcium oxid/hydroxid and air in a slurry. CO2 in the air will be transferred over to because of the high pH and The transferred CO2 will form CaCO3 (limestone). The air above the slurry will have low to zero concentrations of CO2. In the top of the jar - the skimmer sucks in the rather CO2 free air. The jar need also to have en opening out to the external environment - normally to let surplus air from the air pump let out but also to let air with CO2 go into the skimmer if the air pump stops. See figure
This is only an idea - have not tested it yet.
But is it a good idea worth to test or just one of those which should go directly into the trash?
Sincerely Lasse
















