Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
TEST METER FOR TESTING NITRATES
- Thread starter Rick Mathew
- Start date
- Tagged users None
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,390
- Reaction score
- 63,728
I don't see why not ...Beer's Law says it should be so..who am I to argue with him...
My procedure is do the measurement using standard amount (16mL) ...If the reading exceeds the meter range then dilute the tested sample by 1/2 and remeasure...multiply meter results by 2 and plug results into regression formula ...the reaction color appears to be stable for several minutes...seems to work
Just remember (as you likely know), dilution and multiplication also multiplies most types of errors.
Good Morning
I am from portugal and my english is not very good but I have been following your experiences carefully using google translator would it be possible for Mr. Rick Mathew to make a small step by step manual to use the HI-764 CHECKER as a nitrate test?
Thank you for all your efforts in developing the test.
I am from portugal and my english is not very good but I have been following your experiences carefully using google translator would it be possible for Mr. Rick Mathew to make a small step by step manual to use the HI-764 CHECKER as a nitrate test?
Thank you for all your efforts in developing the test.
Just remember (as you likely know), dilution and multiplication also multiplies most types of errors.
Only too aware of this...errors rarely compensate they mostly accumulate ....
Good Morning
I am from portugal and my english is not very good but I have been following your experiences carefully using google translator would it be possible for Mr. Rick Mathew to make a small step by step manual to use the HI-764 CHECKER as a nitrate test?
Thank you for all your efforts in developing the test.
Have you had a chance to checkout the previous posts on this subject ?
rick
I followed the posts from the beginning but I'm afraid that the google translator deceives me that is the reason of my request because I get a little lost
if you can help me please
thanks in advanced
if you can help me please
thanks in advanced
Just remember (as you likely know), dilution and multiplication also multiplies most types of errors.
Of course, but for most of us if my nitrate is 6 or 8 it doesn’t really matter, but I do want to know if it’s 6 or 15.
This method just gives the ULR users with nitrate in the 5-25 range a method rather than having to buy the nitrite meter. A lot of us have the ULR meter already.
I’ll play around with it later today and report back.
Of course, but for most of us if my nitrate is 6 or 8 it doesn’t really matter, but I do want to know if it’s 6 or 15.
This method just gives the ULR users with nitrate in the 5-25 range a method rather than having to buy the nitrite meter. A lot of us have the ULR meter already.
I’ll play around with it later today and report back.
Great...be anxious to know how it work for you
rick
I followed the posts from the beginning but I'm afraid that the google translator deceives me that is the reason of my request because I get a little lost
if you can help me please
thanks in advanced
Will do what I can...Let me know if can follow the document I sent to you...if not we can find a different way
Will do what I can...Let me know if can follow the document I sent to you...if not we can find a different way
Hi, Mr. Mathew
I do not know if I understood correctly but I did not receive any documents from Mr.
Or maybe I did not get it.
thanks any help
thank you
best regards
Careful -- I just might call the sig fig police on you. That's a heck of a lot of decimal places you got going on there.
At present, based on the discussions in this thread, I'm using
[NO3 ppm] = 0.048 [NO2 ppb]
for any meter reading that doesn't require 1:2 dilution, and
[NO3 ppm] = 0.092 [NO2 ppb] + 0.50
for any meter reading that does require dilution (i.e. > 200).
Admittedly, using two equations is a bit arbitrary, but a single linear equation doesn't seem to fit all the data points optimally, and I'm disinclined to do higher level math just to determine my nitrate level. I need something I can ask Alexa to calculate for me.
Hi, Mr. Mathew
I do not know if I understood correctly but I did not receive any documents from Mr.
Or maybe I did not get it.
thanks any help
thank you
best regards
Here is the document I sent you..it was in a different thread called "WATER"
Attachments
Here is the document I sent you..it was in a different thread called "WATER"
The thread was a personal conversation you started with me titled "Water"
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,107
This is great work. This test protocol will give very nice consistent results, with minimal fuss.
Wanted to chime in on discussion about what to do with this intercept of 0.50 - Suggesting zero on the meter should calculate as 0.5ppm NO3. The intercept is meaningful and important.
Don't ditch the intercept!
It's not correct to fit a meter like this to force it to go through 0,0. The regression line should go through zero meter reading at the Limit of Detection.
None of the hanna meters go through 0,0 they all do stuff like this...
Leave the chemical tests out of it - this is just color detection - drops of brown pigment (vanilla) in a cuvette.
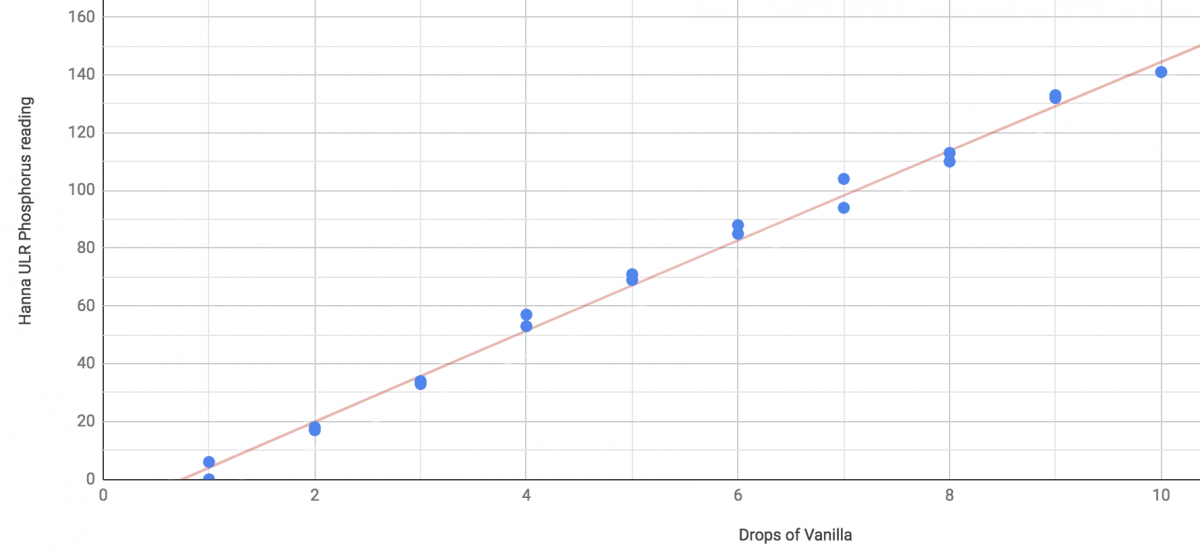
The above is the ULR P meter. As you can see, it'll give approximately zero reading right around 1 drop. nothing below that is detectable, 0,0 is not on the fit line.
Not just that meter either. Silica too...
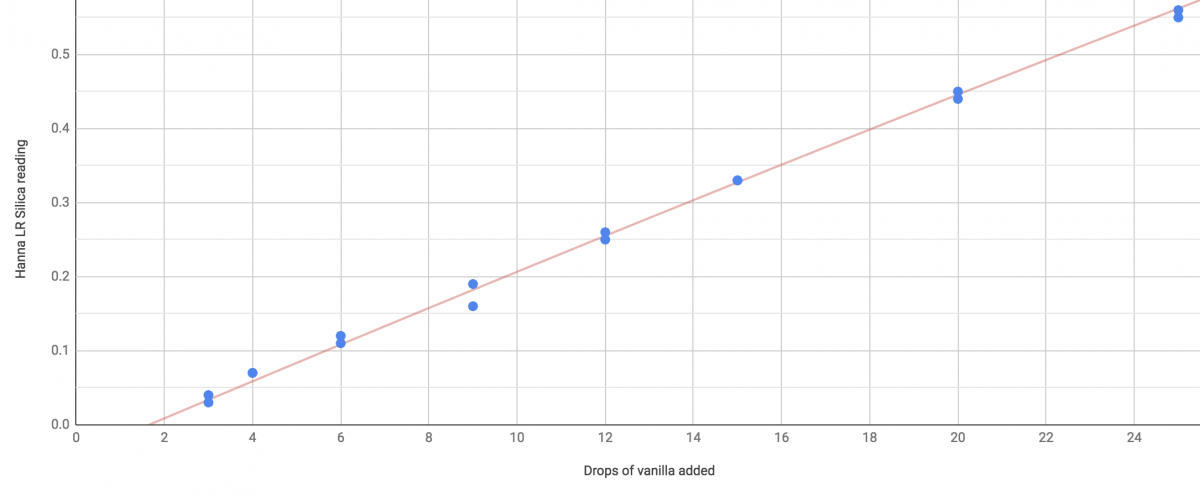
Limit of detection for the LR Si meter is right about 2 drops. again, 0,0 is not on the line. Does a zero meter reading here mean that there's ~2 drops? no it should be understood to mean "2 drops or less"
So how to think of the intercept of 0.5 and a meter reading of zero for the purpose of this thread?
If the meter reading is zero, It's telling you NO3 is around the 0.5ppm Limit of Detection or less. If you need to be able to distinguish between 0.1 and 0.3ppm NO3 (seriously?) then the ULR Nitrite (hi764) is not the meter for it - run the developed color through the ULR P (hi736) meter instead. (And @Rick Mathew or @Dan_P or myself can talk about fit-lines for it.)
Wanted to chime in on discussion about what to do with this intercept of 0.50 - Suggesting zero on the meter should calculate as 0.5ppm NO3. The intercept is meaningful and important.
This first tank raises an interesting concern with this method. The linear equation that best fits the experimental data used to establish this technique, [NO3-ppm] = 0.0458 x [NO2-ppb] + 0.502, does not go through 0,0. However, it should. Otherwise, even a sample with no visible color development and a nitrite meter reading of 0 will still result in a measurement of 0.5 ppm nitrate, which isn't correct.
Don't ditch the intercept!
It's not correct to fit a meter like this to force it to go through 0,0. The regression line should go through zero meter reading at the Limit of Detection.
None of the hanna meters go through 0,0 they all do stuff like this...
Leave the chemical tests out of it - this is just color detection - drops of brown pigment (vanilla) in a cuvette.
The above is the ULR P meter. As you can see, it'll give approximately zero reading right around 1 drop. nothing below that is detectable, 0,0 is not on the fit line.
Not just that meter either. Silica too...
Limit of detection for the LR Si meter is right about 2 drops. again, 0,0 is not on the line. Does a zero meter reading here mean that there's ~2 drops? no it should be understood to mean "2 drops or less"
So how to think of the intercept of 0.5 and a meter reading of zero for the purpose of this thread?
If the meter reading is zero, It's telling you NO3 is around the 0.5ppm Limit of Detection or less. If you need to be able to distinguish between 0.1 and 0.3ppm NO3 (seriously?) then the ULR Nitrite (hi764) is not the meter for it - run the developed color through the ULR P (hi736) meter instead. (And @Rick Mathew or @Dan_P or myself can talk about fit-lines for it.)
Don't ditch the intercept!
Makes sense.
This is great work. This test protocol will give very nice consistent results, with minimal fuss.
Wanted to chime in on discussion about what to do with this intercept of 0.50 - Suggesting zero on the meter should calculate as 0.5ppm NO3. The intercept is meaningful and important.
Don't ditch the intercept!
It's not correct to fit a meter like this to force it to go through 0,0. The regression line should go through zero meter reading at the Limit of Detection.
None of the hanna meters go through 0,0 they all do stuff like this...
Leave the chemical tests out of it - this is just color detection - drops of brown pigment (vanilla) in a cuvette.

The above is the ULR P meter. As you can see, it'll give approximately zero reading right around 1 drop. nothing below that is detectable, 0,0 is not on the fit line.
Not just that meter either. Silica too...

Limit of detection for the LR Si meter is right about 2 drops. again, 0,0 is not on the line. Does a zero meter reading here mean that there's ~2 drops? no it should be understood to mean "2 drops or less"
So how to think of the intercept of 0.5 and a meter reading of zero for the purpose of this thread?
If the meter reading is zero, It's telling you NO3 is around the 0.5ppm Limit of Detection or less. If you need to be able to distinguish between 0.1 and 0.3ppm NO3 (seriously?) then the ULR Nitrite (hi764) is not the meter for it - run the developed color through the ULR P (hi736) meter instead. (And @Rick Mathew or @Dan_P or myself can talk about fit-lines for it.)
Thank you sir...Good point on the intercept.
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,107
Wanted to make up a few known NO3 solutions of my own to test this on. Agreement is really quite good!
Stocks made with zero NO3 35ppt SW. Tested at 1, 2, 3, 7,8, and 11ppm NO3
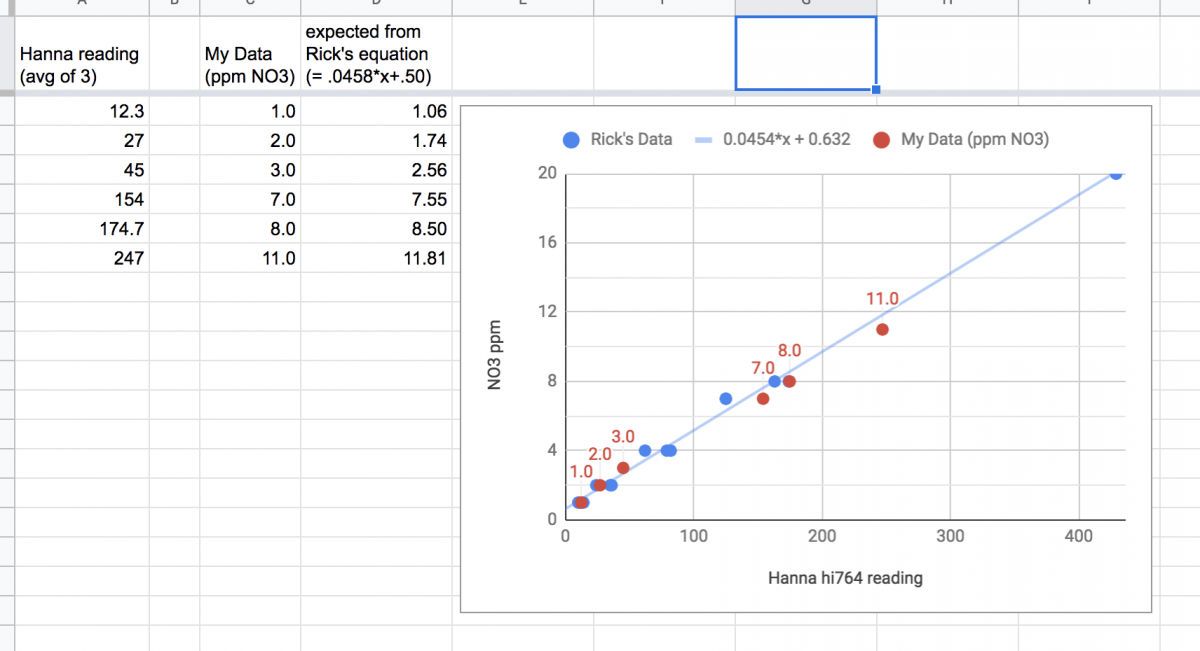
Fantastic work!
Stocks made with zero NO3 35ppt SW. Tested at 1, 2, 3, 7,8, and 11ppm NO3
Fantastic work!
Wanted to make up a few known NO3 solutions of my own to test this on. Agreement is really quite good!
Stocks made with zero NO3 35ppt SW. Tested at 1, 2, 3, 7,8, and 11ppm NO3

Fantastic work!
Thank you sir and thanks for replicating the experiment...this gives us more confidence in our efforts
rick
- Joined
- Sep 21, 2018
- Messages
- 6,684
- Reaction score
- 7,174
Wanted to make up a few known NO3 solutions of my own to test this on. Agreement is really quite good!
Stocks made with zero NO3 35ppt SW. Tested at 1, 2, 3, 7,8, and 11ppm NO3

Fantastic work!
Seeing this correlation is almost better than watching Game of Thrones!
Seeing this correlation is almost better than watching Game of Thrones!
Wow...now that is saying something!!
Similar threads
- Replies
- 6
- Views
- 161
- Replies
- 1
- Views
- 82


















