- Joined
- Aug 24, 2016
- Messages
- 1,504
- Reaction score
- 2,297
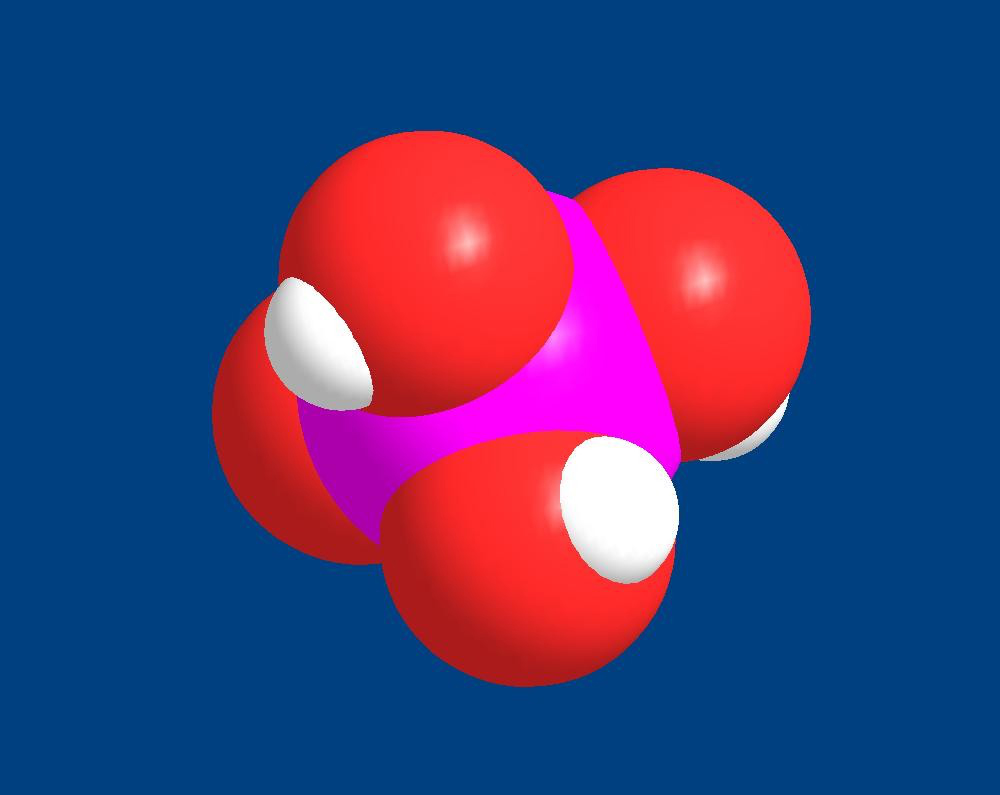
I found the article quite hard to read and understand. Finally for me the only possible contribution to our question of phosphate adsorption to calcium carbonate was:Both polyphosphate and orthophosphate are known to bind well to calcium carbonate surfaces, and are both used in scale control for that reason
Comparing Polyphosphate and Orthophosphate Treatments of Solution-Precipitated Aragonite Powders
The aqueous and thermal stabilities of aragonite (CaCO3) powders against phase conversion are important for industrial applications that rely on calcium carbonate. We describe the synthesis and characterization of solution-precipitated aragonite powders before and after exposure to different...www.mdpi.com
"While mM concentrations are often too low to trigger significant
phosphate mineral formation in the presence of CaCO3 [27,28], higher (mM) phosphate
concentrations have been shown to cause phosphate mineralization [29]. In acidic and
neutral environments, the strong dissolution of calcium carbonate contributes to the interaction
between calcium and phosphate ions, resulting in calcium phosphate precipitates [30].
However, as pH increases, phosphate uptake gradually decreases due to inhibition of
calcium carbonate dissolution; this is consistent with the excess mass trend we see from
TGA data (Table 1), where the excess mass (due in part to phosphate uptake) is largest for
the most acidic orthophosphate treatment (pH 7).
...
Comparing the phase complexity of the orthophosphate-treated powders to the almost unchanged
SHMP-treated powders, it is apparent why polyphosphate is widely used as a
dispersant and anti-flocculant. It is worth noting that our 10 mM treatments are equivalent
to 6 g/L, which is a concentration orders of magnitude higher compared to other recent
studies [32]. Even with the high concentrations, we find that any secondary phosphate
phases are barely detectable with ATR-FTIR spectra, even after annealing. However, its
surface-specific interactions with calcium carbonate are poorly studied.
5. Conclusions
Our work compares aqueous polyphosphate and orthophosphate treatments on aragonite,
using similar phosphate concentrations throughout. Polyphosphate treatments not
only help to prevent against aragonite dissolution during exposure to water, but also
provide a slight increase in the thermal stability of aragonite concerning the conversion
to calcite. In contrast, orthophosphate triggers secondary phases to form during the treatments,
which leads to more complex thermal annealing behaviours."
Doesn't this suggest that uptake of orthophosphate is higher than of polyphosphate and more polyphosphate remains in solution? I have to say I am not sure whether it says that and whether it helps us.