@Randy Holmes-Farley
When does unbalanced Na/Cl-based dosing impact a reef tank?
I've always tried to practice balance dosing to keep my parameters in line with natural or at least within acceptable ranges.
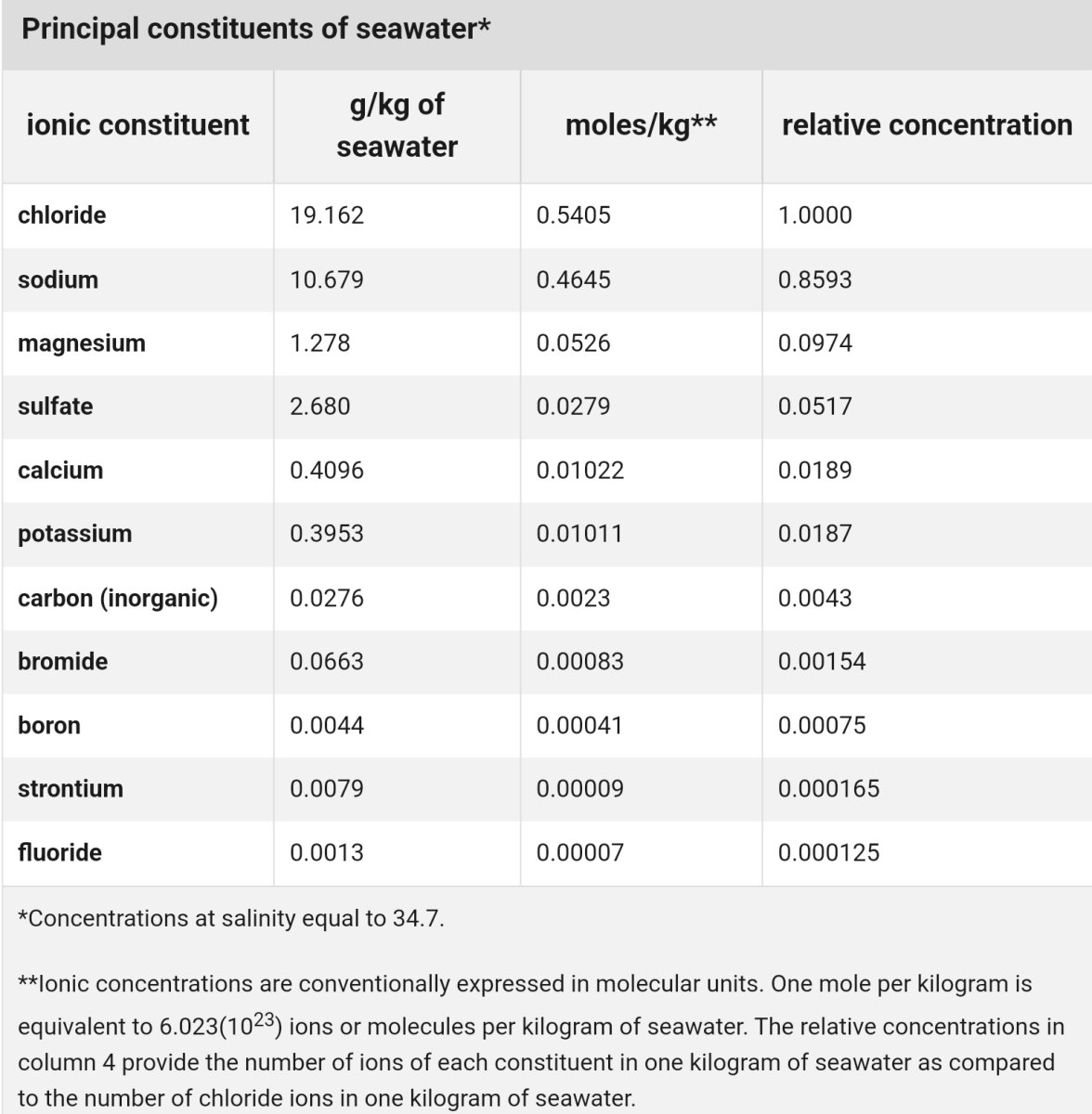
To me balanced dosing = keeping the ionic concentrations.
For instance, typical 2-part dosing = Calcium Chloride + Sodium Carbonate/Bicarbonate.
Since sodium and chloride aren't really depleted in a real tank, dosing 2-part replaces depleted Calcium and Alk...but increases Na and Cl concentration. (3-part strives to fix this by proportionally adding the other missing components. Some even try to account for known depleted trace ions.)
Many other products we dose are Na/Cl based.
As we keep these tanks for many many years, at what point does this type of unbalanced dosing have an impact? Is there a level of increased Na or Cl that would negatively impact the tank?
I know with Kalk dosing (considered balanced dosing), every 6 months or so, I typically need to reduce my Ca as each tank consumes Ca slightly differently based on inhabitants and maturity of the tank as other components can be incorporated into calcification.
When does unbalanced Na/Cl-based dosing impact a reef tank?
I've always tried to practice balance dosing to keep my parameters in line with natural or at least within acceptable ranges.
To me balanced dosing = keeping the ionic concentrations.
For instance, typical 2-part dosing = Calcium Chloride + Sodium Carbonate/Bicarbonate.
Since sodium and chloride aren't really depleted in a real tank, dosing 2-part replaces depleted Calcium and Alk...but increases Na and Cl concentration. (3-part strives to fix this by proportionally adding the other missing components. Some even try to account for known depleted trace ions.)
Many other products we dose are Na/Cl based.
As we keep these tanks for many many years, at what point does this type of unbalanced dosing have an impact? Is there a level of increased Na or Cl that would negatively impact the tank?
I know with Kalk dosing (considered balanced dosing), every 6 months or so, I typically need to reduce my Ca as each tank consumes Ca slightly differently based on inhabitants and maturity of the tank as other components can be incorporated into calcification.













