Nice info !Interesting, if I understand the manual correctly it says that it actually tops up the reference reservoir with tankwater if it gets low. "For daily operation, it will adjust water level to target level automatically." in the section about doing waterchanges to the reservoir. There's no inlets or outlets except the tankwater inlet and the entirely separate doser.
And for the reservoir waterchange it then presumably pumps the reservoir content into your tank? That's not nice for nano or pico tanks, but then again neither is returning a pH 4 sample from a titrator.
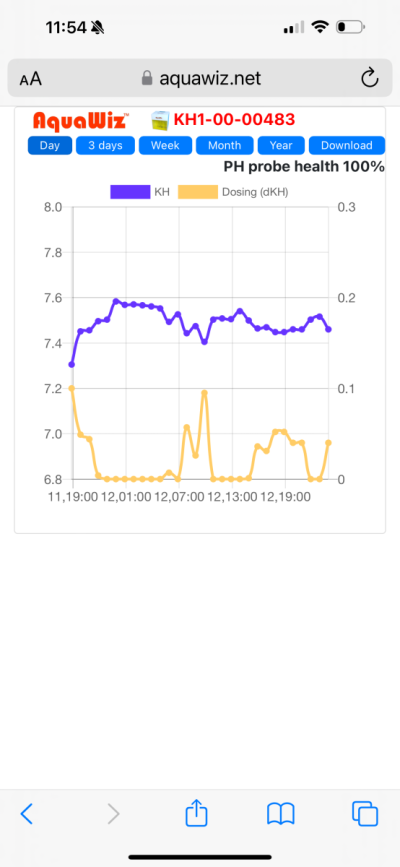
Bit curious how that fits with the claim that evaporation effects on calibration can be neglected.
But who knows, the whole thing seems like one big feedback loop, maybe it all finds an equilibrium longterm
If I can get the pH parts I have on hand to give at least 0.002 precision with some averaging and stuff then I think I'll build one, only thing I'm missing is tiny airstones and an extra air pump I can build into it for recirculation
This suggests to me that one could improve long-term stability massively by introducing a RODI water cleaning back to the tank and ATO process into the reference.
That way one could probably also use room air for the aeration, as the increased evaporation losses can be offset with the ATO. Additionally one would not need to clean the salt from the air pump.
I wonder why they didn’t go that route, when the device is already at 800$. Suggests to me that I’m overlooking something.
Does alkalinity evaporate with the water when one aerates a sample for a (very) long time, as it seems to do with salt (the reason for cleaning the pump) ?