- Joined
- May 20, 2020
- Messages
- 12
- Reaction score
- 7
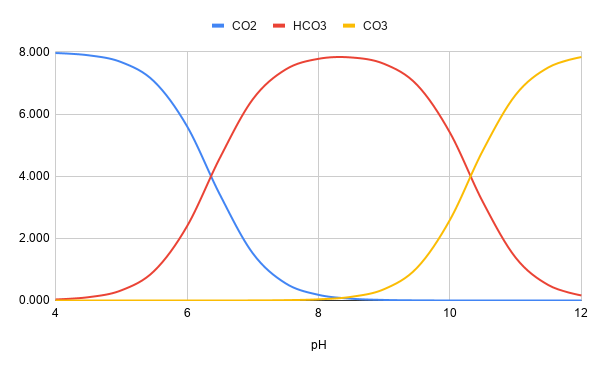
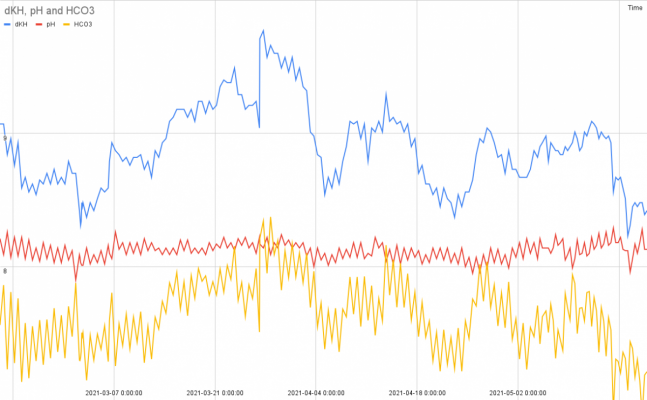
reason is that if its not worth it, its just waste of energy to chase a stable pH, (that was your main message). We are searching the truth, aren’t we, so thats why we discuss. Ofcourse pic up C is the easy way out, but then we have not answered the questions and dont know more than before.I cannot really read the picture to tell what it is. There is no simple experiment that can distinguish bicarbonate from carbonate uptake, hence the reason it has been unclear in the scientific literature. One cannot move them independently without also changing pH. I do not know which they use, and they may use both, or they may use just one.
I'm not sure why one would want to pick between A or B. Your choice C that is better than either (both stable), and another might also be better (stable bicarbonate).