- Joined
- Mar 30, 2016
- Messages
- 789
- Reaction score
- 433
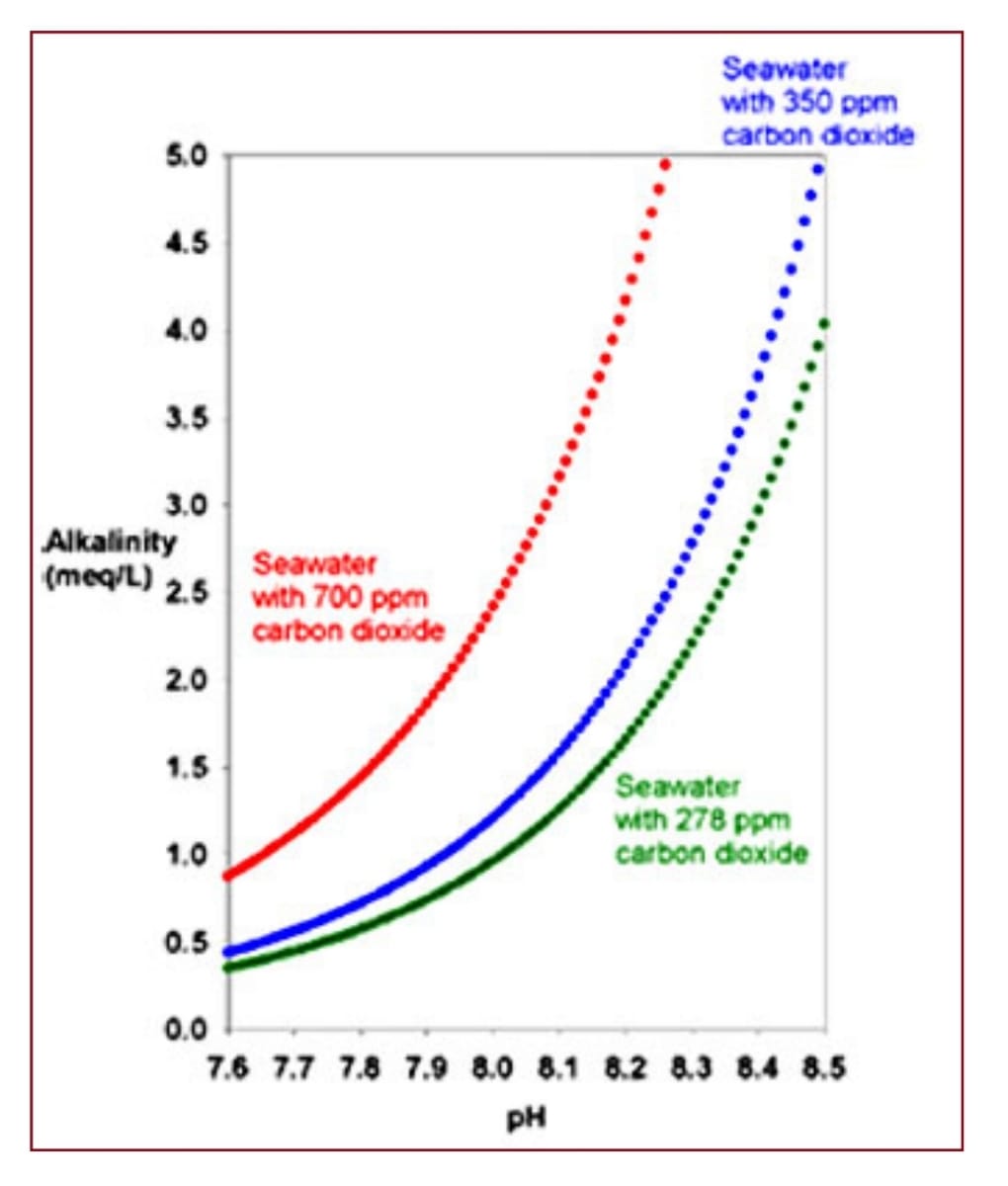
Explain how to move CO2 from the red curve to the green and blue curve...
What mechanisms can be used?
What mechanisms can be used?
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.

As it is mine. And I am recognized in my industries as one of the authorities in water management.Certainly there is. But the chemistry of gases dissolving in water is well within my scientific expertise.
Obviously, this forum is one of your only strongholds in society, Randy.
I will allow this thread to continue and bow out of this fruitless dialogue.
The "law of physics" which I was referring to is the Pauli exclusion principle, which states that two identical fermions (particles with half-integer spin) cannot occupy the same quantum state simultaneously. This applies to normal matter, which is made out of only a few kinds of fermions tightly bonded together.
That includes gases in a given solution.
Gas has mass and gas has a volume, estimated by Avogadro's number and the IDEAL gas law.
In deference, Randy, please show us, that all other engineers and physicists are wrong, in our stating that displacement of gases in a solution is real.
We need facts, not opinions and "because I said so" arguments.


Explain how to move CO2 from the red curve to the green and blue curve...
What mechanisms can be used?

Are you still trying to prove Henry’s law wrong by showing my own graphs that support it?
This is a waste of time for everyone involved.
Nothing you have posted suggests that gases compete for space when air dissolves in water.

Henry's law is based on assumptions and constraints of classical ideal gas laws.
This world is not ideal and cannot be constrained.
So this aeration with lower co2 air... what are the mechanics behind this?As I state in many articles, aerating with lower CO2 air lowers CO2 in the water. That’s exactly what Henry’s law states.

lolIf gas dissolves in water, does it not have mass anymore? Or does it disappear like magic?

Finally, a breakthrough, if you are paying attention.So this aeration with lower co2 air... what are the mechanics behind this?
How does lower co2 air just magically replace higher levels of co2 trapped and dissolved in the water?
So this aeration with lower co2 air... what are the mechanics behind this?As I state in many articles, aerating with lower CO2 air lowers CO2 in the water. That’s exactly what Henry’s law states.
You stated low co2 aeration drives down co2 in the water column.lol
Your point is what?
Obviously, this forum is one of your only strongholds in society, Randy.

So this aeration with lower co2 air... what are the mechanics behind this?
How does lower co2 air just magically replace higher levels of co2 trapped and dissolved in the water?
You stated low co2 aeration drives down co2 in the water column.
I'm asking you HOW? By what physical means? Where did the excess co2 go?

I didn’t address this since I’m not a braggart, but folks who are new here can see my technical and scientific achievements on Linked In. They include inventing pharmaceuticals that members or their families may be taking.Unnecessary.
True... lolUnnecessary.
s
I explained this well understood phenomenon above. It equilibrates into the low CO2 air bubbles and is carried away.
I
I didn’t address this since I’m not a braggart, but folks who are new here can see my technical and scientific achievements on Linked In. They include inventing pharmaceuticals that members or their families may be taking.

So the mechanics are hypothesized and reaching equilibrium is observed.
This means that there still are holes in your argument.
So, low co2 air in, co2 rich air out...s
I explained this well understood phenomenon above. It equilibrates into the low CO2 air bubbles and is carried away.
