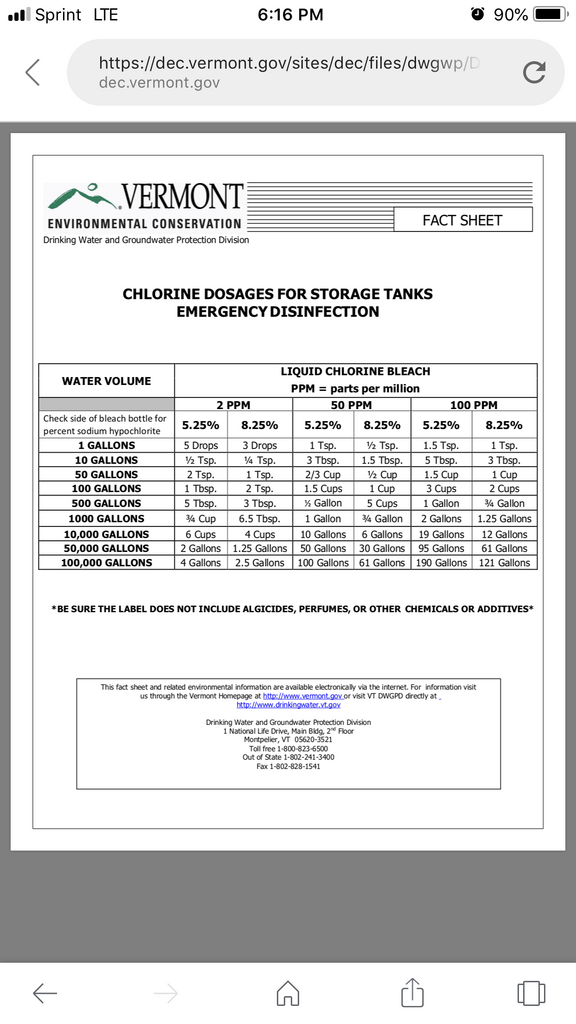
When you clean and sterilize a QT tank...it's been said it's best to fill it up with cold water rather than hot water if you're using bleach. Hot water apparently decomposes the active ingredient of bleach and renders it ineffective.?
My question is...would bleach be changed at all if it's used in saltwater rather than freshwater?
We're trying to figure out the correct dose to use to sterilize 10 gallons of water. Then i'm wondering if the water would play a role in potency if it's saltwater or freshwater. It may not matter...I don't know.
My last question is...does bleach nuke everything, or do you guys think there is a possibility that some parasites, organisms, dinoflagettes, etc that would survive?
My question is...would bleach be changed at all if it's used in saltwater rather than freshwater?
We're trying to figure out the correct dose to use to sterilize 10 gallons of water. Then i'm wondering if the water would play a role in potency if it's saltwater or freshwater. It may not matter...I don't know.
My last question is...does bleach nuke everything, or do you guys think there is a possibility that some parasites, organisms, dinoflagettes, etc that would survive?