I may be misunderstanding but in the time stamped link below from Frag Garage channel, "Episode 15: Claude Schuhmacher - Fauna Marin" Claude Schuhmacher seems to dismiss the idea that Soda Ash provides any meaningful benefit to pH, at least, "not for [a] long time." Is there merit to this? Can anyone explain? @Randy Holmes-Farley Are we all doing it wrong?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Claude Schuhmacher suggests, long term, Soda Ash lowers pH. Any truth to this?
- Thread starter Skep18
- Start date
- Tagged users None
Not having time to watch, but iirc generally only hydroxide additions will provide longer (still under 24hrs) Ph gain. Disregarding alkalinity change, the temporary Ph gain is from converting CO2 to carbonate and freeing some O2. This causes a temporary disparity versus the natural CO2 level in the tank and will eventually equalize around the level of the surrounding air. If your goal is to maintain alkalinity at set level x, say 8.0, then depending on your alkalinity consumption rate you will only see the small Ph hits during the times of dosing. With hydroxide you're doing more conversions on the way to creating the carbonate which requires more CO2, thus driving Ph up faster (inverse relationship).
For anyone reading, be extra mindful when dealing with hydroxide solutions as they are generally very high Ph and can instantly sopanify the oils on your skin (best case scenario). Also requires specific type of plastic (forget the # off the top of my head) as containers need to be very chemically resistant.
For anyone reading, be extra mindful when dealing with hydroxide solutions as they are generally very high Ph and can instantly sopanify the oils on your skin (best case scenario). Also requires specific type of plastic (forget the # off the top of my head) as containers need to be very chemically resistant.
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
He's arguing the pH elevation advantage of carbonate over bicarbonate doesn't last for a long time, as it'll equalize. He also suggests that the equalization over time is smaller with bicarbonate, so he likes it for that stability reason.at least, "not for [a] long time."
Lots and lots of people prefer the higher pH alk additives even accepting that over time the pH boosts decrease as equalization with atmospheric CO2 occurs.
I may be misunderstanding but in the time stamped link below from Frag Garage channel, "Episode 15: Claude Schuhmacher - Fauna Marin" Claude Schuhmacher seems to dismiss the idea that Soda Ash provides any meaningful benefit to pH, at least, "not for [a] long time." Is there merit to this? Can anyone explain? @Randy Holmes-Farley Are we all doing it wrong?
The fact that using high pH additives does usually increase the pH of the tank overall is just a symptom of tanks not being fully equilized to atmospheric CO2. My tank gets to 8.5 regularly, for example. If it were fully aerated it shouldn't exceed 8.3ish given my Alkalinity.
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
This is true, and we normally think of anything that's not in equilibrium as temporary and not ideal.The fact that using high pH additives does usually increase the pH of the tank overall is just a symptom of tanks not being fully equilized to atmospheric CO2.
But we need to think differently about CO2 equilibrium, because the CO2 equilibrium with saltwater is so slow, alk additives often have such high pH and indoor air has so much CO2, that our systems are basically never in CO2-equilibrium. So it's fine to run the system counting on the fact that we can dose high pH additives around the clock and keep our tanks on the low-CO2-side of equilibrium 24/7. It's weird to try to keep that in mind and remember that aggressive aeration works against that - if that is your goal - you don't want CO2 equilibrium.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,780
Just reiterating what folks in this thread have said...
It is unquestionable that using carbonate causes a pH boost, and hydroxide causes a bit over twice the pH boost, while bicarbonate produces a slight pH lowering, all for the same total alkalinity added.
It is certainly true that any tank with good aeration is always driving the tank toward equilibrium with the air, and if you alter the pH by adding or consuming CO2 (which is what these additives do), then the pH will start to move back toward equilibrium with the air. Thus, all of these effects do not last forever, and how fast they drift away will depend on the degree of aeration.
If you primary goal is unchanging pH, then you would use a mix of mostly bicarbonate and a little carbonate, with the exact ratio depending on the pH that you want to hold at. More bicarbonate relative to carbonate if you are trying to hold at a lower pH.
That said, there's no evidence that I have seen that an absolutely steady pH is desirable. It does not happen in nature, and there is pretty strong evidence that higher pH leads to faster hard coral growth rates, while pH too low stresses corals.
IMO hard corals will do best in the 8.2 to 8.5 pH range. They are OK in the pH 7.8 to 8.2 range, and are stressed below pH 7.7. It is somewhat unclear whether going above pH 8.5 is desirable or undesirable from this perspective.
It is unquestionable that using carbonate causes a pH boost, and hydroxide causes a bit over twice the pH boost, while bicarbonate produces a slight pH lowering, all for the same total alkalinity added.
It is certainly true that any tank with good aeration is always driving the tank toward equilibrium with the air, and if you alter the pH by adding or consuming CO2 (which is what these additives do), then the pH will start to move back toward equilibrium with the air. Thus, all of these effects do not last forever, and how fast they drift away will depend on the degree of aeration.
If you primary goal is unchanging pH, then you would use a mix of mostly bicarbonate and a little carbonate, with the exact ratio depending on the pH that you want to hold at. More bicarbonate relative to carbonate if you are trying to hold at a lower pH.
That said, there's no evidence that I have seen that an absolutely steady pH is desirable. It does not happen in nature, and there is pretty strong evidence that higher pH leads to faster hard coral growth rates, while pH too low stresses corals.
IMO hard corals will do best in the 8.2 to 8.5 pH range. They are OK in the pH 7.8 to 8.2 range, and are stressed below pH 7.7. It is somewhat unclear whether going above pH 8.5 is desirable or undesirable from this perspective.
Thanks everyone for the responses! Certainly what I'm hearing is what I've always heard. Nothing new. I just found it shocking that a company such as Fauna Marin would seemingly be against the use of soda ash. Was just curious if anyone understood the perspective enough such that the general community might agree with it. It sounds like not, which again, inherently seems counter intuitive seeing as he owns a company and we're all hobbyists on forums. lol. But I will have to find a way to survive with that disparity...
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,780
I would add to the above discussion even the pH stability argument is negligible if one doses the alk supplements spread out through the day. Then there are many tiny boosts that end up with stable pH (from the dosing) while pH will be variable from photosynthesis and respiration (as usual).
I would add to the above discussion even the pH stability argument is negligible if one doses the alk supplements spread out through the day. Then there are many tiny boosts that end up with stable pH (from the dosing) while pH will be variable from photosynthesis and respiration (as usual).
I would not want to ask you outright come out against Claude's comments but I'm not able to come up with any thought process that seems plausible as to the proposed mindset... Even more odd is the claim the sodium bicarbonate has a higher long term (whatever that means) pH benefit...
I need a chemistI would not want to ask you outright come out against Claude's comments but I'm not able to come up with any thought process that seems plausible as to the proposed mindset... Even more odd is the claim the sodium bicarbonate has a higher long term (whatever that means) pH benefit...
"The formula for sodium bicarbonate is NaHCO3. There is another compound called washing soda or sodium carbonate, which has the formula Na2CO3. Heating baking soda drives off the hydrogen and gives you washing soda. Dissolving baking soda yields one sodium ion and one bicarbonate anion (HCO3–).
Dissolving washing soda gives you two sodium ions and one carbonate ion (CO32-)."
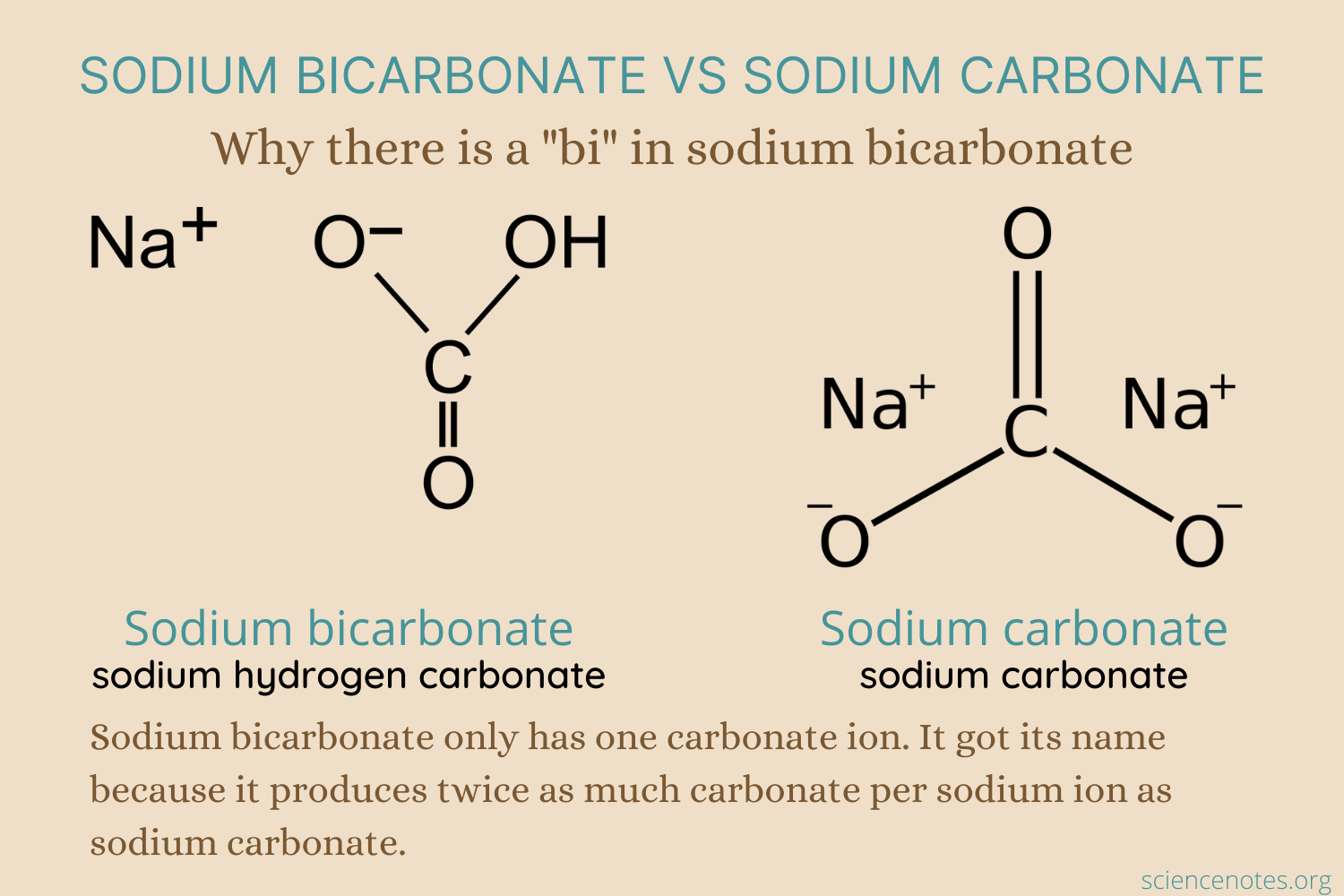
Why Is Baking Soda Called Sodium Bicarbonate?
Have you ever wondered why baking soda is called sodium bicarbonate, even though it only contains one carbonate? Here is the answer.
“So let it be written, so let it be done”
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,780
There is no possible scenario where dosing bicarbonate leads to a higher pH than dosing the same amount of alk with carbonate to reach the same alkalinity.
Likewise, there is no possible scenario where dosing carbonate leads to a higher pH than dosing the same amount of alk with hydroxide.
Precipitation of calcium carbonate makes the analysis more complicated, but at the same final alk in the water, the above statements are always true.
Likewise, there is no possible scenario where dosing carbonate leads to a higher pH than dosing the same amount of alk with hydroxide.
Precipitation of calcium carbonate makes the analysis more complicated, but at the same final alk in the water, the above statements are always true.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,780
I need a chemist
"The formula for sodium bicarbonate is NaHCO3. There is another compound called washing soda or sodium carbonate, which has the formula Na2CO3. Heating baking soda drives off the hydrogen and gives you washing soda. Dissolving baking soda yields one sodium ion and one bicarbonate anion (HCO3–).
Dissolving washing soda gives you two sodium ions and one carbonate ion (CO32-)."
Probably happens !
Why Is Baking Soda Called Sodium Bicarbonate?
Have you ever wondered why baking soda is called sodium bicarbonate, even though it only contains one carbonate? Here is the answer.sciencenotes.org
“So let it be written, so let it be done”
OMG, isnt the internet great? lol
You do not drive off hydrogen, you drive off carbon dioxide and water.
2NaHCO3 —> H2O + CO2 + Na2CO3
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,780
It would be more fun if you drove off hydrogen, then your oven might explode...
Yes, that would be exciting!
Similar threads
- Replies
- 2
- Views
- 325
- Replies
- 6
- Views
- 569
- Replies
- 6
- Views
- 385
-
- AMS: Article
- Replies
- 61
- Views
- 4,152