Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,946
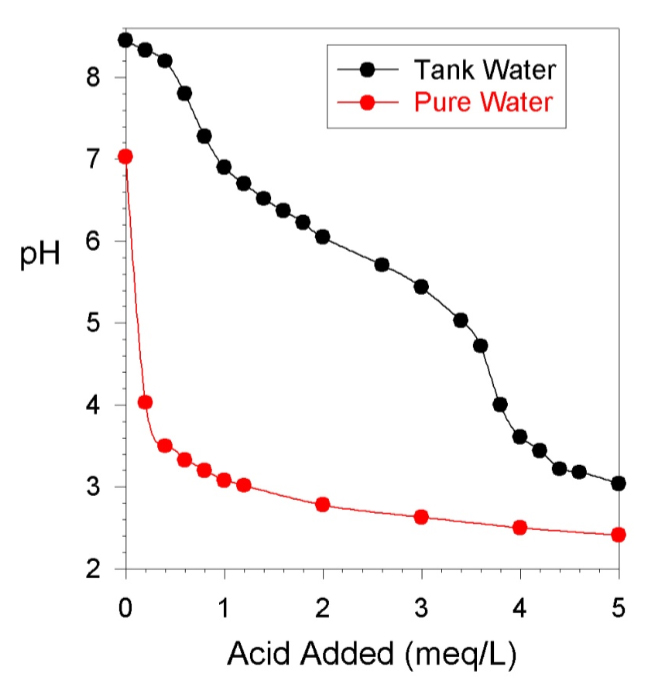
Used a Pyrex 1 cup measuring cup. Filled it to the 1 cup line as close as I could.
I think a Pyrex measuring cup could easily be off by 10%.