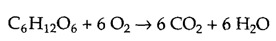Agree on definition. But then maybe urea could be organic carbon dosing because metabolisation of whole molecule urea, not only its "carbon" part could be energetically net positive, at least for some algae as some of the abovementioned papers suggested.I agree. That’s how I would define organic carbon dosing: adding an organic material that provides energy when it is metabolized. I’ve not calculated the energetics, but it may be low and not that useful for this purpose.
Meaning also dosing urea could be bad for reef tanks because will stimulate more algae growth than growth of heterotroph bacteria.