I know Randy's strong opinion that zeolites are useless in salt water and they are serving mostly as substrate for bacteria  but in this particular case I'm not fully agree. Actually zeolites (and particularly sodium–potassium forms like clinoptilolite) do work in salt water - they do remove Ammonia from it. Yes, the excess of Na+ and K+ in sea water do influence ammonia removal negatively and zeolites in sea water are 30-40% less effective if compared to distilled water, but this could be adjusted by adding more zeolites. And they are removing Ammonia quite quickly in about 20 min.
but in this particular case I'm not fully agree. Actually zeolites (and particularly sodium–potassium forms like clinoptilolite) do work in salt water - they do remove Ammonia from it. Yes, the excess of Na+ and K+ in sea water do influence ammonia removal negatively and zeolites in sea water are 30-40% less effective if compared to distilled water, but this could be adjusted by adding more zeolites. And they are removing Ammonia quite quickly in about 20 min.
Here is very good reading with results from US EPA team's research:
https://www.acc.umu.se/~vatten/zeolite.pdf
Here is graph from it showing dependence of zeolite Ammonia removal capacity from salinity (i've modified slightly for easier understanding, red dot is salinity @35)
In the same paper there are results showing zeolites could remove 100% of Copper, Lead and Zinc from sea water, which explain why Zeovit method is using copper containing supplements like ZeoSpur2 without constrains that copper will accumulate in the water column. .
.
So it is true, the Zeovit method is using zeolites as main exporter of nutrients, especially of nitrogen. That's why I have bag of Zeovit stones in hand in case of ammonia peak in my thank or metal contamination.
Here is very good reading with results from US EPA team's research:
https://www.acc.umu.se/~vatten/zeolite.pdf
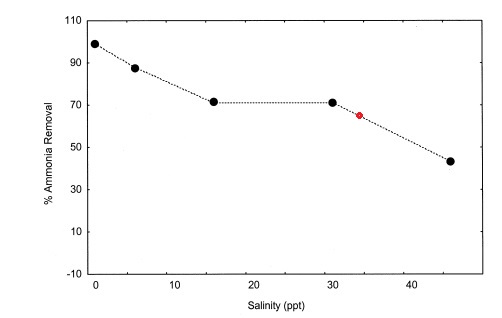
Here is graph from it showing dependence of zeolite Ammonia removal capacity from salinity (i've modified slightly for easier understanding, red dot is salinity @35)
In the same paper there are results showing zeolites could remove 100% of Copper, Lead and Zinc from sea water, which explain why Zeovit method is using copper containing supplements like ZeoSpur2 without constrains that copper will accumulate in the water column.
So it is true, the Zeovit method is using zeolites as main exporter of nutrients, especially of nitrogen. That's why I have bag of Zeovit stones in hand in case of ammonia peak in my thank or metal contamination.

















