- Joined
- May 22, 2016
- Messages
- 6,542
- Reaction score
- 10,099
Prime certainly dechlorinates. That is easily measurable and verifiable. It's a strong reducer. It has saved many fish from tap water. (just not from ammonia) 
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
Yes, it works as a dechlorinator. This was the first thing I checked when I first observed that it did not affect the ammonia level.Prime certainly dechlorinates. That is easily measurable and verifiable. It's a strong reducer. It has saved many fish from tap water. (just not from ammonia)
Correct.@Dan_P @taricha Thanks guys. I must admit, I was following the amphipod experiment predominantly, so missed a lot of other info.
To summarize - so we found out how Seachem Prime actually allegedly detoxifies anmonia, and that the allegedly detoxified ammonia is definitely not supposed to be registrable with non-Nessler/salicylate tests, so by measuring free ammonia with them we found no significant changes - is that right?
Great perspective that challenges our thinking about ammonia. For many aquarists, it will take nerves of steel not to reach for the bottle of Prime when they think their aquarium is having an ammonia event.Let me try to address a recurring question - "but Prime saved my fish from an ammonia event?"
Let me give a brief (haha) run-down of some reasons why a hobbyist might think that Prime etc has saved their livestock from ammonia when it hasn't. (No judgment, less than a year ago, I had some fish loss and not much time, detected a little ammonia, and dosed Prime to "save" the unaffected fish. I was convinced it had worked. Here's why I was wrong.)
1. Ammonia accumulates more slowly than we think. When I feed generously for my small fish, (pinch of flake + cube of mysis + cube of brine) I am only putting in enough protein to elevate total ammonia by 0.5 ppm.
Since fish release ~80% of protein input as ammonia, this would take 5 days to reach 2ppm ammonia. If you feed sparingly, you could probably stretch that out to 2 weeks before total ammonia would go up to 2 ppm, even if your tank consumed zero.
2. Most ammonia is non-toxic NH4. NH3 is a small %. pH really matters. Even if I have the hypothetical 2ppm total ammonia at pH 8.2, then it's only 0.13ppm toxic NH3. see calculator. If the pH drops to 7.8 then 2ppm total ammonia is only 0.05ppm NH3.
3. Most organisms are tougher than the few sensitive fish we think about. From RHF article
" Marine fish generally have 96 h LC50 levels that range from about 0.09 to 3.35 ppm NH3-N."
That is, while there are sensitive fish that have a LC50 (lethal concentration to 50% of specimens) over 4 days (96 hr) of ~ 0.1ppm NH3, many saltwater fish are much tougher. So many systems could spend days at the above hypothetical 2ppm total ammonia (pH 8.2, NH3 = 0.13) and not lose livestock. Benthic organisms are even tougher than that, in looking up NH3 tolerances for random inverts in my system, pods, asterinas, snails, crabs, shrimp - all quite high by our sensibilities.
4. We assume new fish will be a little stressed in a new environment "take a few days to settle down." Many hobbyists might confuse some sub-lethal level of NH3-stress for new to a system stress, and think Prime prevented NH3 toxicity.
5. Any non-frozen bottle of traditional nitrifying bacteria can keep up with a slow rise in ammonia laid out the scenario in numbers 1 and 2. Recommended dose of Biospira can eat ~0.5ppm ammonia/day straight out of the bottle (for me anyway). One and Only a little slower, Fritz turbostart a bit faster. They expand capacity quite well too, especially if there is a constant presence of ammonia.
6. Heterotrophic bacteria are everywhere (and in some bottled tank starter products). They can reproduce really fast if conditions are right. With addition of carbon - present in all fish food - they can process ammonia quickly also. Plenty fast to avoid bad outcomes. Bottled bacteria myth or fact thread showed repeatedly the headscratching result that adding fish food caused ammonia to go down with these bacteria.
7. There are a zillion photosynthetic organisms that will show up rapidly in any tank that has light. Photosynthetic organism are strong consumers of ammonia themselves, and they release dissolved organic carbon that can help the heterotrophs consume ammonia also.
8. Photosynthetic organisms have such a strong preference for ammonia, that they have a hard time consuming any other form of N, if any ammonia is present. They'll ignore 20ppm NO3 to grab 0.2ppm ammonia. It's not like they are choosing it, it's just so much more energetically favorable as a food source.
9 / Putting it all together.
Tank "crashes" / die-offs don't raise NH3 as much as you might think. A 100g of dead fish that hides in your 55 gal (210 Liter) tank doesn't immediately turn into a bunch of ammonia. The fish might be ~20% protein, ~16% of protein is N, so 100*.20*.16 = 3.2g N. 3200mg N / 210 Liters = 15mg/L N = ~19 ppm total ammonia
Protein is broken down gradually over days to a week so maybe only 3ppm ammonia release per day. Some of that is eaten by clean up crew, that might assimilate 20% into growth and release the other 80% as ammonia. The carbon in the tissues also increases heterotroph activity that reduces how much N is released into ammonia. And on top of that, the metabolic processes involved in breaking down the fish will lower pH as well, thus reducing the fraction of ammonia that is NH3.
If all that decomposing organic material pushes the pH down to say 7.8 (my tank goes that low sometimes without dead fish), then the daily 3ppm total ammonia release would be only 0.08ppm NH3.
And that's without even considering the nitrifiers and the photosynthesizers, that with ammonia present will do nothing but eat ammonia, day and night.
So when I lost a couple of small fish to rapid disease and added Prime to "protect" the rest from toxic ammonia, there was never actually a clearly toxic condition to protect them from.
If I wanted to do something I could be sure would help protect fish during an ammonia event:
1) lower pH (7.8 is fine in a pinch, I wouldn't try to push the aragonite buffer around ~7.6)
2) add algae
3) add carbon (vinegar or whatever carbon you already use in your system)
4) add aeration (heterotrophs and nitrifiers both need O2 to work well, and the die-off could push O2 low.)
edit: 5) and y'know... water changes
Those are all incontrovertible and do not require trusting any unsupported manufacturer claims.
To summarize - so we found out how Seachem Prime actually allegedly detoxifies anmonia, and that the allegedly detoxified ammonia is definitely not supposed to be registrable with non-Nessler/salicylate tests, so by measuring free ammonia with them we found no significant changes - is that right?
Yeah. The chemistry tests run at multiple concentrations of ammonia and prime (one in my first post and a bunch in Dan's post number 16) are the heart of the story.Correct.

To summarize - so we found out how Seachem Prime actually allegedly detoxifies anmonia,
I mean you did affirm my summary, I presumed that meant you guys actually found out how Prime was supposed to allegedly detoxify ammonia. I didn't respond later exactly because reading through your posts again, I could not find any info. But decided to leave it be.Things seemed to have gotten a little twisted around
Our data says Prime does not reduce ammonia content and therefore does not detoxify ammonia.

 www.reef2reef.com
www.reef2reef.com
I tested Ammonia and Red Sea NO3 tests with thiosulfate (similar to Prime - some assert that Prime active ingredient turns into thiosulfate) and I found that the tests are very much interfered with by thiosulfate.Does adding Prime change ammonia test kit read report accuracy even if prime itself isn’t impacting free ammonia?
Prime dechlorinator is very similar to thiosulfate, which I found a tiny bit of to completely wipe out an ammonia and NO2/3 test.

tiny amount of thiosulfate zero'd out the color of both API ammonia, and Red Sea NO3.
Prime likely interferes with chemical kits, which complicates measurement and obscures what it does/doesn't do.
Probably not relevant but may as well put it out there. I tried to get a false nitrite reading with different stuff and couldn’t get it to read anything apart from nowt;I tested Ammonia and Red Sea NO3 tests with thiosulfate (similar to Prime - some assert that Prime active ingredient turns into thiosulfate) and I found that the tests are very much interfered with by thiosulfate.
I wanted to do some checking before I said this also for sure happens with Prime. It does.

left pic: Red Sea NO3 pro with ~0.7ppm NO3, top is normal, bottom is with 2x Prime.
center pic: Red Sea marine NO2/NO3 test kit, Nitrite test, sample water has ~1ppm ammonia and ~30ppm NO3. Neither without (top) or with (bottom) 2x Prime does it show any false NO2.
right: API ammonia at 5min with 1ppm ammonia, left tube is normal, right tube is with 2x Prime.
These were done 5 min after addition of Prime. I checked again at 45 min and it still interfered with API total ammonia. I do not know how many hours the interference lasts for.
With Prime, cycling tests are unreliable. The strong reducing agent causes a negative interference with total ammonia and NO3 tests, and probably also NO2 tests (because the final step of an NO3 test is an NO2 test), but I have no NO2 samples to demonstrate that with. I didn't have an API NO2 test handy, but given that it zeros out NO3 tests, I don't see how Prime could positively interfere with an NO2 test to show fake NO2.
(I haven't gone through the specifics of that thread to comment on it)
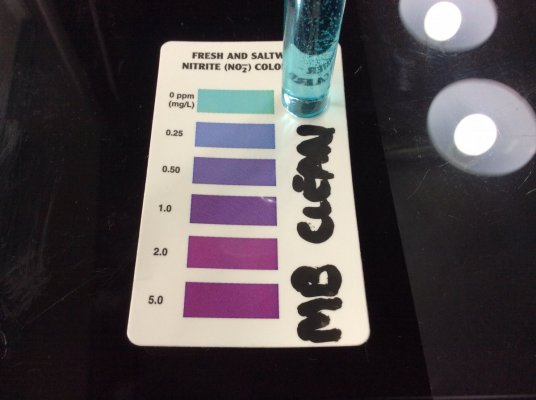

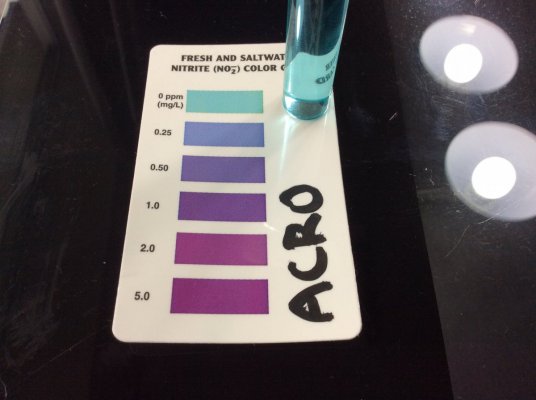

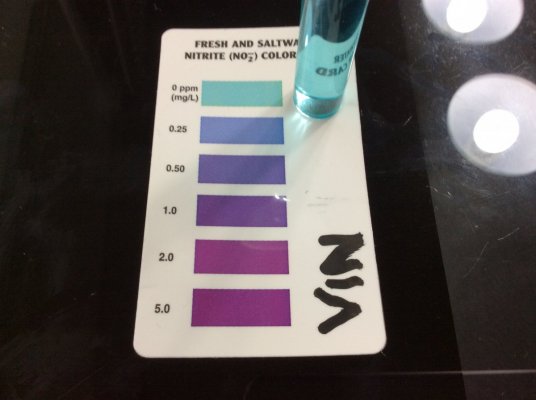

Nitrate and nitrite use the same chemistry. The only difference is the extra step in the nitrate test where nitrate is reduced to nitrite with zinc. So yes, if Prime interferes with the nitrate test, it will interfere with the nitrite test.I tested Ammonia and Red Sea NO3 tests with thiosulfate (similar to Prime - some assert that Prime active ingredient turns into thiosulfate) and I found that the tests are very much interfered with by thiosulfate.
I wanted to do some checking before I said this also for sure happens with Prime. It does.

left pic: Red Sea NO3 pro with ~0.7ppm NO3, top is normal, bottom is with 2x Prime.
center pic: Red Sea marine NO2/NO3 test kit, Nitrite test, sample water has ~1ppm ammonia and ~30ppm NO3. Neither without (top) or with (bottom) 2x Prime does it show any false NO2.
right: API ammonia at 5min with 1ppm ammonia, left tube is normal, right tube is with 2x Prime.
These were done 5 min after addition of Prime. I checked again at 45 min and it still interfered with API total ammonia. I do not know how many hours the interference lasts for.
With Prime, cycling tests are unreliable. The strong reducing agent causes a negative interference with total ammonia and NO3 tests, and probably also NO2 tests (because the final step of an NO3 test is an NO2 test), but I have no NO2 samples to demonstrate that with. I didn't have an API NO2 test handy, but given that it zeros out NO3 tests, I don't see how Prime could positively interfere with an NO2 test to show fake NO2.
(I haven't gone through the specifics of that thread to comment on it)
Thank you. We saw this before we designed our experiments. We both used the Seachem free ammonia sensing colorimetric films and I used the Seneye device to look for the reduction in free ammonia by Prime, which we failed to observe. Ironic that a Seachem product invalidates another Seachem product.Apologies if someone already mentioned this, but the FAQ says...
I am using Prime® to control ammonia but my test kit says it is not doing anything, in fact it looks like it added ammonia! What is going on?
A: A Nessler based kit will not read ammonia properly if you are using Prime®... it will look "off scale", sort of a muddy brown (incidentally a Nessler kit will not work with any other products similar to Prime®). A salicylate based kit can be used, but with caution. Under the conditions of a salicylate kit the ammonia-Prime® complex will be broken down eventually giving a false reading of ammonia (same as with other products like Prime®), so the key with a salicylate kit is to take the reading right away. However, the best solution ;-) is to use our MultiTest™ Ammonia kit; it uses a gas exchange sensor system which is not affected by the presence of Prime® or other similar products. It also has the added advantage that it can detect the more dangerous free ammonia and distinguish it from total ammonia (total ammonia is both free ammonia and non-toxic ionized forms of ammonia).
@brandon429I didn't have an API NO2 test handy, but given that it zeros out NO3 tests, I don't see how Prime could positively interfere with an NO2 test to show fake NO2.
It seems hilariously likely that the positive claims around Prime about ammonia, nitrite and nitrate detoxification as well as the criticisms that Prime prevents cycling bacteria may all be based on the simple fact that it breaks a lot of hobby test kits, and makes them show zero incorrectly.So yes, if Prime interferes with the nitrate test, it will interfere with the nitrite test.
