Hi
I thought I know most things affecting the pH in a reef aquarium with a very heavy photosynthesis - but I do not
Look at this chart. You see a daily variation of the pH depending on photosynthesis and CO2 production/consumption. From 20:00 in the evening to noon the next day I´m dosing the Core 7 3A and 3B. Around 16:00 the 19/6 I discover that I had low calcium levels - around 340 ppm. I decide to use CaCl *2 H2O in order to adjust the levels. The normal dose of core 7 (1,2 3A and 3B) continue. I dose an onetime dose directly and set one of my dosing pumps to dose 32 times a day and with a amount that will rise the Ca level with 40 ppm in 7 days. and look at the graph - what´s happen. The only change is the dosing of CaCl*2H2O. Light is the same. Can it be a fast calcification that alter the pH? Not more CO2 in the room. I have normally 400 - 800 ppm CO2 in my air - these last days - no higher than 640 ppm
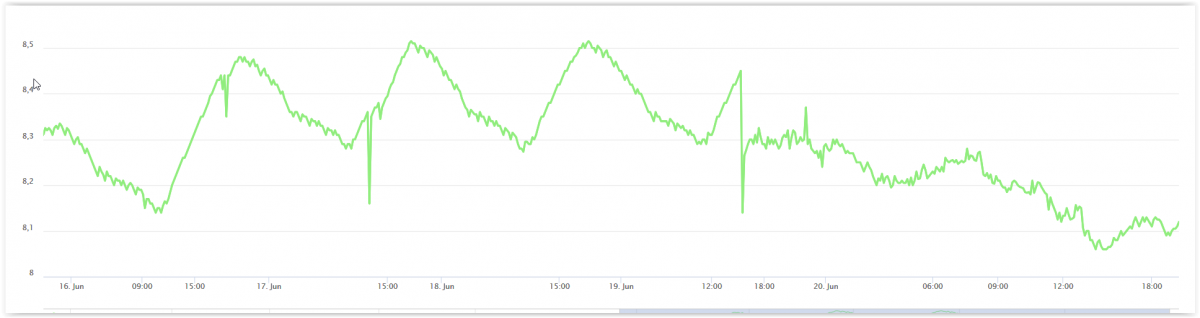
Yesterday KH 8.6 - today 8. Calcium - when I discover the problem 330 - today 350 ppm
Sincerely Lasse
I thought I know most things affecting the pH in a reef aquarium with a very heavy photosynthesis - but I do not
Look at this chart. You see a daily variation of the pH depending on photosynthesis and CO2 production/consumption. From 20:00 in the evening to noon the next day I´m dosing the Core 7 3A and 3B. Around 16:00 the 19/6 I discover that I had low calcium levels - around 340 ppm. I decide to use CaCl *2 H2O in order to adjust the levels. The normal dose of core 7 (1,2 3A and 3B) continue. I dose an onetime dose directly and set one of my dosing pumps to dose 32 times a day and with a amount that will rise the Ca level with 40 ppm in 7 days. and look at the graph - what´s happen. The only change is the dosing of CaCl*2H2O. Light is the same. Can it be a fast calcification that alter the pH? Not more CO2 in the room. I have normally 400 - 800 ppm CO2 in my air - these last days - no higher than 640 ppm
Yesterday KH 8.6 - today 8. Calcium - when I discover the problem 330 - today 350 ppm
Sincerely Lasse
Last edited:



















