I had sent in a water sample just to see how my parameters were. Everything came back normal except for phosphorus(which I already knew was high), low strontium, and high salinity. The one that I question was the salinity. Before I sent the water sample off I tested the normal parameters and the salinity came to be 35ppt (and I just calibrated the refractometer)but the test came back at 37.59ppt. I messaged the company and they said “Hello Nick, our ICP is calibrated with NIST standards and we actually add up all the elements to give you a true value of parts per thousand. A refractometer is not as accurate as an environmental instrument designed to detect every element in the salt water.”
My question is, should I trust the 37.59ppt? If I do, when I use my refractometer should I actually be looking for 32-33ppt which would be within that 2.59ppt difference?
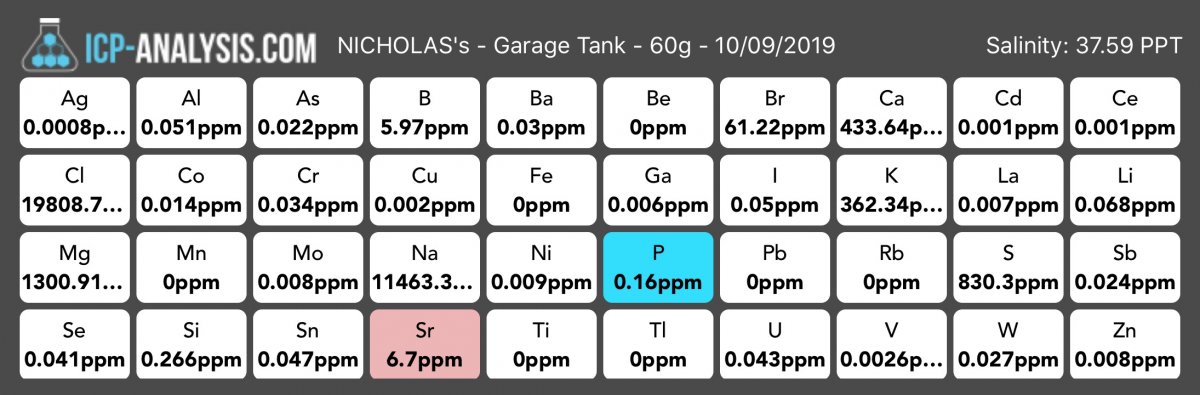
My question is, should I trust the 37.59ppt? If I do, when I use my refractometer should I actually be looking for 32-33ppt which would be within that 2.59ppt difference?
















