Hello,
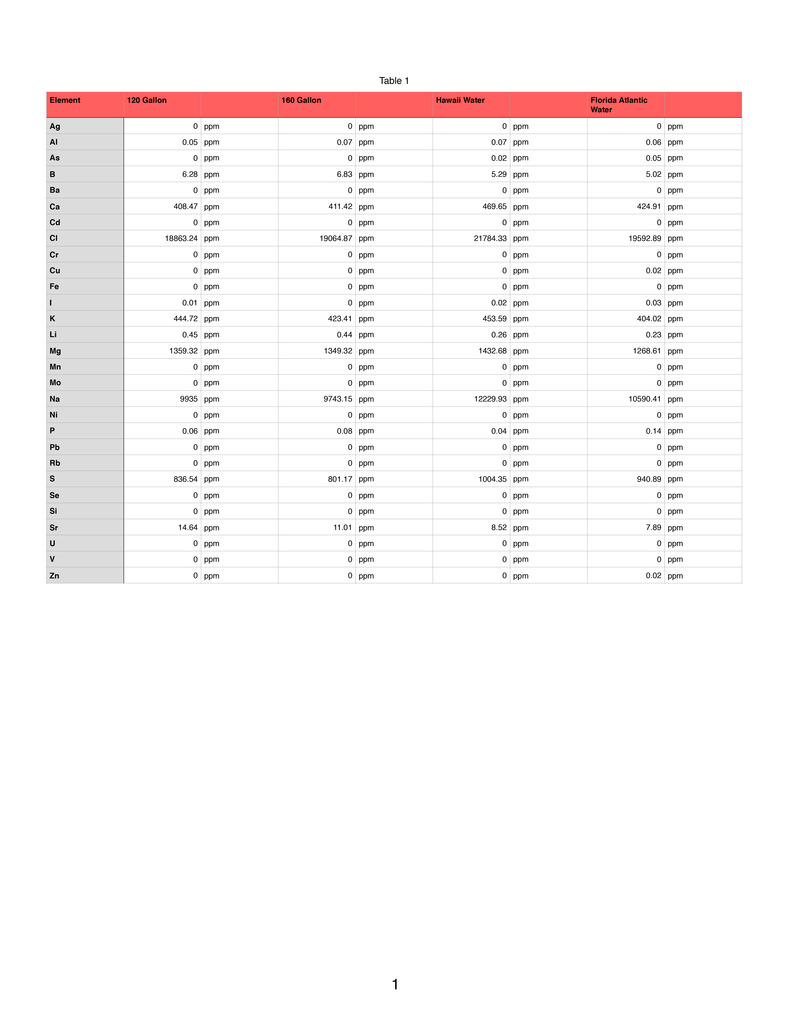
I had my water tested by ICP-Analysis.com on Monday and another sample 3 months ago was sent to Aquaforest. The minor trace element values vary greatly, should I take the new sample results with a grain of salt? I have been dosing Seachem Reef Trace as well as Seachem Reef Plus at one cap per week for each tank so there should be some amount of minor trace elements reading in the new test.
ICP-Analysis.com Results
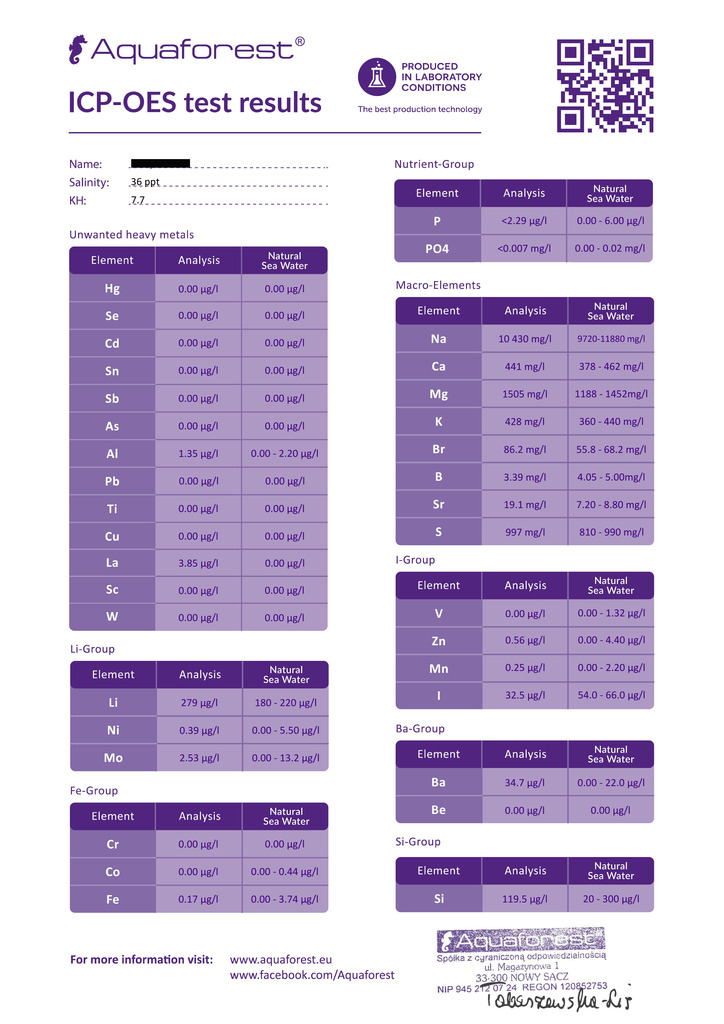
Aquaforest Results
120 Gallon
160 Gallon
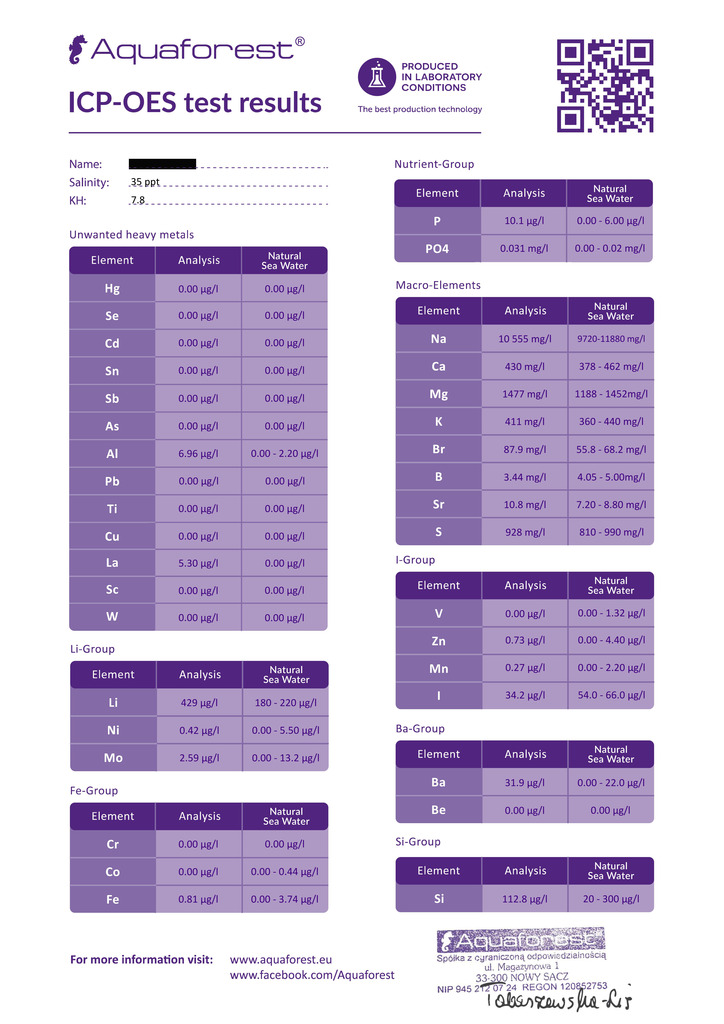
I had my water tested by ICP-Analysis.com on Monday and another sample 3 months ago was sent to Aquaforest. The minor trace element values vary greatly, should I take the new sample results with a grain of salt? I have been dosing Seachem Reef Trace as well as Seachem Reef Plus at one cap per week for each tank so there should be some amount of minor trace elements reading in the new test.
ICP-Analysis.com Results

Aquaforest Results
120 Gallon

160 Gallon
















