There is another older video that explains this better. I use the balling method in my tank and I double the amount of part C. So if you use 50 ml of alkalinity daily you would dose 100 ml Part C.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Lowering high salinity with TM Part C (Balling)
- Thread starter Vested
- Start date
- Tagged users None
@Hans-Werner How do I change your equation to work for a tank with a different amount of total gallons? So say its a 250 gallon tank and I still want to go from 38psu to 35psu.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
@Hans-Werner How do I change your equation to work for a tank with a different amount of total gallons? So say its a 250 gallon tank and I still want to go from 38psu to 35psu.
I'm sure Hans-Werner will answer too, but the amounts of everything involved will scale exactly with the size of the tank, so a 100 gallon tank will take twice as much as a 50 gallon tank.
Where does the 11% come from, so for the 450 I figured out 11% of the water would be 50 gallons assuming the salinity of the part c water is 11ppt.I'm sure Hans-Werner will answer too, but the amounts of everything involved will scale exactly with the size of the tank, so a 100 gallon tank will take twice as much as a 50 gallon tank.
for example
I have a 200gallon tank that is at 37psu and I want 35psu
2g/l x 43% = 0.86
Dose 0.86g per liter tank volume
0.86 x 757.082
Dose 651.09052g of part c to how much ro water?
Ok I think I mis-understood and actually the equation is assuming the ro waterchange is pure ro 0ppt like how it would be dosed normally. So same example following the equation..
I have a 200gallon tank that is at 37psu and I want 35psu
2g/l x 43% = 0.86
Dose 0.86g per liter tank volume
0.86 x 757.082
Dose 651.09052g of part c to 15.5 gallons of ro water assuming this waterchange is all at once
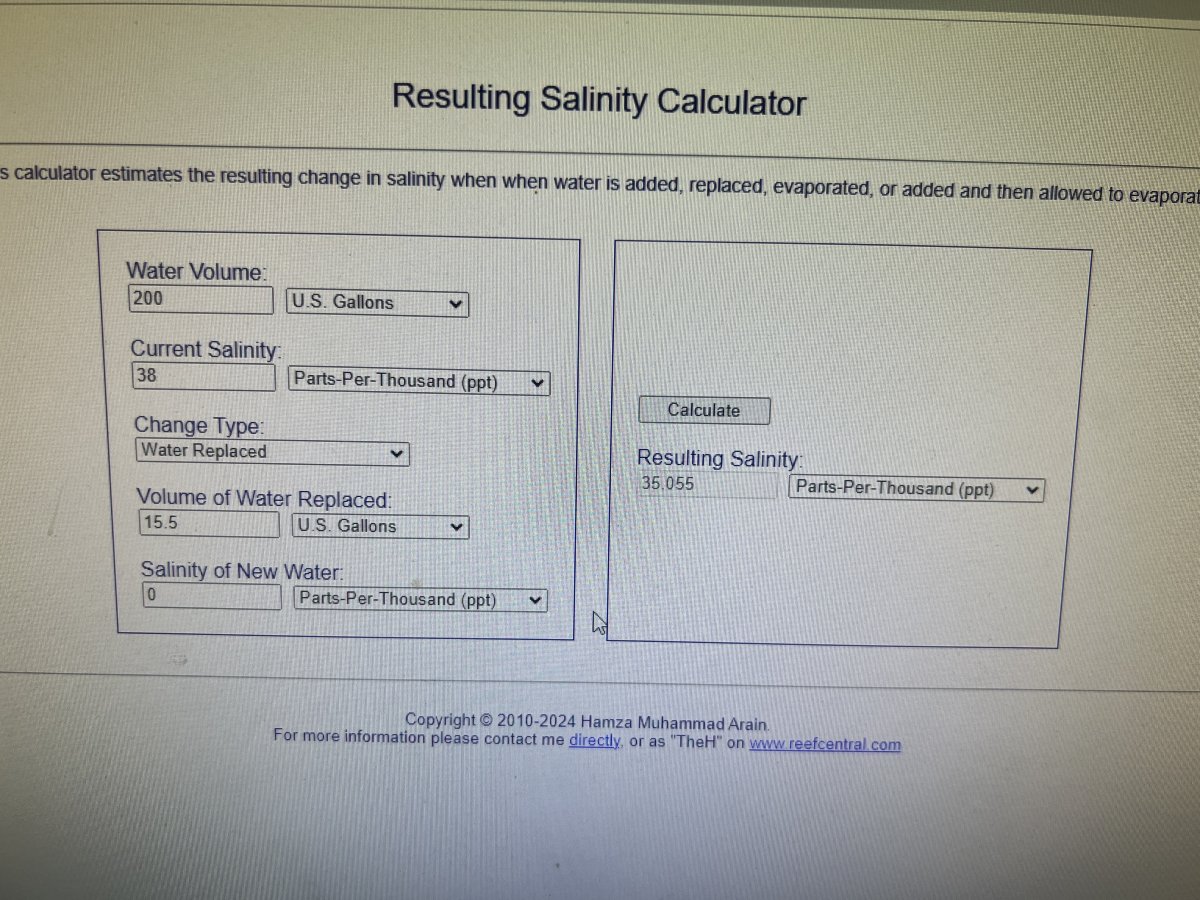
Now I dont want todo it all at once and would then have to account for the percentage each time which is gonna get really complicated.
Could I mix 651.09052g of part c into a random 5 gallon jug dose that to the tank and then slowly reduce the salinity by removing water and replacing with 0ppt ro water. I guess the question is what amount of part c is safe to dose all at once and how diluted does it need to be to not precipitate?
I have a 200gallon tank that is at 37psu and I want 35psu
2g/l x 43% = 0.86
Dose 0.86g per liter tank volume
0.86 x 757.082
Dose 651.09052g of part c to 15.5 gallons of ro water assuming this waterchange is all at once
Now I dont want todo it all at once and would then have to account for the percentage each time which is gonna get really complicated.
Could I mix 651.09052g of part c into a random 5 gallon jug dose that to the tank and then slowly reduce the salinity by removing water and replacing with 0ppt ro water. I guess the question is what amount of part c is safe to dose all at once and how diluted does it need to be to not precipitate?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
OMG, why did you ask about a different tank volume and salinity than what you wanted? Wasted effort. 
Rule 1 in how to ask questions: don't ask fake questions
Rule 1 in how to ask questions: don't ask fake questions
Not a fake question and not wasted effort I have multiple tanks at different salinities and volumes and im trying to understand how to solve this with that equation.OMG, why did you ask about a different tank volume and salinity than what you wanted? Wasted effort.
Rule 1 in how to ask questions: don't ask fake questions
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
The calculator is suitable for such calculations. It matches my calculation, for example.
If you split it up into multiple removals and additions, then you need to use the calculator multiple times.
If you split it up into multiple removals and additions, then you need to use the calculator multiple times.
The calculator is suitable for figuring out the salinity change assuming you know the salinity of the makeup water. Which I cant figure out unless hans equation is assuming its zero which I think it is now? Thats where the confusion wasThe calculator is suitable for such calculations. It matches my calculation, for example.
If you split it up into multiple removals and additions, then you need to use the calculator multiple times.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
I deleted my calculation post because I used the wrong salinity for Balling Part C.The calculator is suitable for figuring out the salinity change assuming you know the salinity of the makeup water. Which I cant figure out unless hans equation is assuming its zero which I think it is now? Thats where the confusion was
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
edited: see changes below
Last edited:
That was said nowhere in this entire thread how am I suppost to know to mix it to 10.5 and how does that line up with the changing amount of grams based on the equations hans sent lol im so confused.
So now I need to mix up a random amount of balling part c to 10.5 ignore the equation and use the water change calculator to figure out how much to replace to get to 35?
So now I need to mix up a random amount of balling part c to 10.5 ignore the equation and use the water change calculator to figure out how much to replace to get to 35?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
That was said nowhere in this entire thread how am I suppost to know to mix it to 10.5 and how does that line up with the changing amount of grams based on the equations hans sent lol im so confused.
So now I need to mix up a random amount of balling part c to 10.5 ignore the equation and use the water change calculator to figure out how much to replace to get to 35?
That's by far the easiest way, IMO.
Perhaps I can get that info out of what Hans-Werner has already written.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
I think I need to think this through a bit more. I'm confusing myself and writing nonsense.
Lol I feel ya, late to work trying to figure it out all morning. Appreciate itI think I need to think this through a bit more. I'm confusing myself and writing nonsense.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
The Tropic Marin Original Balling Part C is for a 70 : 30 ratio, 70 % sodium chloride and 30 % Part C sodium chloride free sea salt. Starting from sodium chloride only as in your case, you have to add 43 % of the assumed sodium chloride excess in Part C.
Example: You have an excess of 3 g/l of sodium chloride. 3 g x 43 % = 1.3. You have to add 1.3 g per liter or 4.9 g per US-gallon in Part C while reducing salinity of the tank water.
In total the excess of sea salt now would be 3 g/l + 1.3 g/l = 4.3 g/l. This excess is sufficient to supply sea salt (normal dosage for 35 PSU is 39 g/l or 3.9 %) for 0.11 l to achieve ca. 35 PSU salinity.
This means you can remove 11 % of saltwater and s l o w l y add 11 % reverse osmosis water with the calculated amount (4.9 grams per US-gallon water volume) of Part C.
Since you are removing 11 % of the tank water you can subtract this from the Part C also, so take just 4.4 grams of Part C per US-gallon of water volume.
By the way: Very interesting article on salinity and Goniopora in the BRS video. It seems to confirm my experience that a slightly reduced salinity of 33 PSU is at least as good or better than 35 PSU for corals. In fact in the tropical coasts where most corals grow the salinity is also slightly reduced to ca. 34 PSU due to heavy rainfalls.
OK, I think we are good to go now.
I'm showing Hans-Werner's calculation above, and working from it.
The 3 g/l excess sodium chloride he uses comes from the salinity being 38 ppt, when you want 35 ppt. (that assumes you got to 38 ppt by starting at 35 ppt and using an unbalanced two part to add sodium chloride).
If you have a different high salinity value, such as 37 ppt, you alter that 3 g/L number (say, to 2 g/L for 37 ppt starting value).
The amount of Balling Part C you want to add is 30/70 x this value, or 30/70 x 3 g = 1.3 g/L. If the value of excess sodium chloride is smaller (say the 2 g/L mentioned above, then it is 30/70 x 2 = 0.85 g/L Balling Part C.
The L values in the 1.3 g/L or 0.85 g/L represents the total liters in your aquarium, not the amount you are going to change.
Thus, for 250 gallons = 946 liters, you would be adding (in total) 1.3 g/L x 946 L = 1230 grams of Balling Part C.
That amount fixes the ionic composition in the 250 gallons, but not the salinity.
If all that was added at once (dry), then the salinity would rise a bit above 38 ppt (38 g/L + ~1 g/L) = 39 ppt (I'm estimating the amount of water already in Balling Part C, it will be off a bit)
Then use the calculator to figure out how much RO/DI would need to swap out from ~39 ppt to get to 35 ppt, and put all the Balling part C into that volume and change away.
- Joined
- Dec 28, 2016
- Messages
- 22,829
- Reaction score
- 21,964
If your salinity rose. All of the other elements should have risen as well. Right?That will work if your water changing with it but you're also lowering every other element in the water unnaturally so you will be deficient once back to 35ppt. The theory is that adding part c to the RO/DI water you replace it should have traces to make up for it?
- Joined
- Dec 28, 2016
- Messages
- 22,829
- Reaction score
- 21,964
Curious would that be because the potassium is being used up more quickly. It seems that if the salinity is 38 the potassium etc would also be high. Depending on what caused the 38 in the first place?That’s not what I meant by fully balanced. It may certainly be adequate, but ions such as potassium can still become depressed by salinity corrections.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
FWIW< that final calculation with the calculator is about where the 11% came from. 89% 39 ppt + 11% 0 ppt --> 34.7 ppt
Similar threads
- Replies
- 8
- Views
- 127
- Replies
- 10
- Views
- 154
- Replies
- 1
- Views
- 95
- Replies
- 3
- Views
- 321
-
- AMS: Article
- Replies
- 61
- Views
- 4,140


















