What were the results of your cup aeration tests?
I haven't performed the tests yet. We got another winter storm and I am waiting for that to clear up before going outside. Will try and do them tomorrow.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
What were the results of your cup aeration tests?
Aquatrance 3000s Max air 880L/H according to https://www.coralvue.com/aquatrance-3000s-skimmer-pump-aq-3000s
vrs
2400L/H with a Varos 6 according to here - https://reefoctopus.com/product/octo-empty/
Could be a step in the right direction putting a differant pump on it & why your pH is still so low with what you've already done.
I'm of the view the old school sps guys liking oversize skimmers & kalk was really because of the pH benefit it gave but they didn't realise it at the time.
Turn your skimmer off sometime & watch what your pH does to appreciate what its doing.
Vrs the airstones setup i don't know but with some decent air pull thru a skimmer i'd be surprised that the airstones would win
Edit:
Or what about this:
On 1 side your scrubbing with the airstones but on the other your putting it back in with non scrubbed skimmer air? Maybe worth a try adding a scrubber into that skimmer line.
Forgot to post my overnight results.Daytime was running 7.92 but waited until morning just before turning lights on to test PH and it was 8.9 something. Don't recall exactly yet it seems the algae base I have isn't performing enough photosynthesis to be the cause of why my PH has not gone up from averaging around 7.5. Assuming flow wasn't the catalysis depending on house CO2 then could it be due to maturing denitrification filter. Been trying to research the possibility that heterotrophic bacteria might utilize bound oxygen when nitrates and sulfates have been exhausted or better understand how denitrification exactly increases base. Thought it was purely a chemical process as with acidification during nitrification but could the bacteria be pulling the oxygen molecule from carbon dioxide therefore leaving behind carbonate. Would partially explain the rise in alkalinity although I now believe much of that due to my melting coral skeletons (Reborn) during nitrification. Very puzzling and hoping to figure this out.Photosynthesizing algae (or anything) will raise the daytime pH.
Cycling depletes alk and lowers pH.
You may be confusing #s in your post of pH and dKH.Forgot to post my overnight results.Daytime was running 7.92 but waited until morning just before turning lights on to test PH and it was 8.9 something. Don't recall exactly yet it seems the algae base I have isn't performing enough photosynthesis to be the cause of why my PH has not gone up from averaging around 7.5. Assuming flow wasn't the catalysis depending on house CO2 then could it be due to maturing denitrification filter. Been trying to research the possibility that heterotrophic bacteria might utilize bound oxygen when nitrates and sulfates have been exhausted or better understand how denitrification exactly increases base. Thought it was purely a chemical process as with acidification during nitrification but could the bacteria be pulling the oxygen molecule from carbon dioxide therefore leaving behind carbonate. Would partially explain the rise in alkalinity although I now believe much of that due to my melting coral skeletons (Reborn) during nitrification. Very puzzling and hoping to figure this out.

Been trying to research the possibility that heterotrophic bacteria might utilize bound oxygen when nitrates and sulfates have been exhausted or better understand how denitrification exactly increases base. Thought it was purely a chemical process as with acidification during nitrification but could the bacteria be pulling the oxygen molecule from carbon dioxide therefore leaving behind carbonate. Would partially explain the rise in alkalinity although I now believe much of that due to my melting coral skeletons (Reborn) during nitrification. Very puzzling and hoping to figure this out.
I'm not confusing it. Well versed in both.You may be confusing #s in your post of pH and dKH.
It's virtually impossible to have a pH of 7.5 in a reef tank unless your tank has poor aeration, you are dosing something that lowers pH, or you house C02 is so high that it's unhealthy for humans and would cause health issues with extended exposure. If none of the above is true, you have a measurement issue.
In terms of Alk, your dKH will deplete as NO3 increases. However, once your tank matures and NO3 is consumed, that Alk will be returned to the tank.
I'm not confusing it. Well versed in both.
New build. No corals. No dosing. No supplementation. Just lots of media performing both nitrification and denitrification. Using an HM Digital PH-200 that is often calibrated and have never found it to not be calibrated. No clue how much CO2 in my house but will get around to buying a method of measuring it. Thought it might be an aeration issue but Randy brought up a good point that may disqualify that as to why it went up. Only change I made was increasing the flow through my filtration which is the same process which creates flow at the surface. Hence why I thought in my situation it PH went up. Has in the past. Nitrates did rise. Seems denitrification might have been affected by the increased flow and the most logical explanation.
My DKH is stable at 11. Top off is distilled water. Flow has been reduced and will test again.
I'm not confusing it. Well versed in both.
New build. No corals. No dosing. No supplementation. Just lots of media performing both nitrification and denitrification. Using an HM Digital PH-200 that is often calibrated and have never found it to not be calibrated. No clue how much CO2 in my house but will get around to buying a method of measuring it. Thought it might be an aeration issue but Randy brought up a good point that may disqualify that as to why it went up. Only change I made was increasing the flow through my filtration which is the same process which creates flow at the surface. Hence why I thought in my situation it PH went up. Has in the past. Nitrates did rise. Seems denitrification might have been affected by the increased flow and the most logical explanation.
My DKH is stable at 11. Top off is distilled water. Flow has been reduced and will test again.
That's enlightening. Thought alkalinity lost during nitrification was not fully returned during denitrification which seemed odd to me since I thought of it as energy where it can only be transferred and not created or consumed.Consumption of nitrate adds alkalinity whether it is denitrifiation or simple consumption to form tissue.
I show the detailed process here:
Alkalinity Decline in the Nitrogen Cycle
One of the best known chemical cycles in aquaria is the nitrogen cycle. In it, ammonia excreted by fish and other organisms is converted into nitrate. This conversion produces acid, H+ (or uses alkalinity depending on how one chooses to look at it), as shown in equation 1:
(1) NH3 + 2O2 --> NO3- + H+ + H2O
For each ammonia molecule converted into nitrate, one hydrogen ion (H+) is produced. If nitrate is allowed to accumulate to 50 ppm, the addition of this acid will deplete 0.8 meq/L (2.3 dKH) of alkalinity.
However, the news is not all bad. When this nitrate proceeds further along the nitrogen cycle, depleted alkalinity is returned in exactly the amount lost. For example, if the nitrate is allowed to be converted into N2 in a sand bed, one of the products is bicarbonate, as shown in equation 2 (below) for the breakdown of glucose and nitrate under typical anoxic conditions as might happen in a deep sand bed:
(2) 4NO3- + 5/6 C6H12O6 (glucose) + 4H2O --> 2 N2 + 7H2O + 4HCO3- + CO2
In equation 2 we see that exactly one bicarbonate ion is produced for each nitrate ion consumed. Consequently, the alkalinity gain is 0.8 meq/L (2.3 dKH) for every 50 ppm of nitrate consumed.
Likewise, equation 3 (below) shows the uptake of nitrate and CO2 into macroalgae to form typical organic molecules:
(3) 122 CO2 + 122 H2O + 16 NO3- --> C106H260O106N16 + 138 O2 + 16 HCO3-
Again, one bicarbonate ion is produced for each nitrate ion consumed.
It turns out that as long as the nitrate concentration is stable, regardless of its actual value, there is no ongoing net depletion of alkalinity. Of course, alkalinity was depleted to reach that value, but once it stabilizes, there is no continuing alkalinity depletion because the export processes described above are exactly balancing the depletion from nitrification (the conversion of ammonia to nitrate).
There are, however, circumstances where the alkalinity is lost in the conversion of ammonia to nitrate, and is never returned. The most likely scenario to be important in reef aquaria is when nitrate is removed through water changes. In that case, each water change takes out some nitrate, and if the system produces nitrate to get back to some stable level, the alkalinity again becomes depleted.
House temps at 72, tank at 78 and outside currently at 54. It's literally impossible for me to perform the cup test. Best I just buy a home air quality measuring tool. I feel fine around my tank. Still getting and air exchanger at some point.7.5 NBS with 11dKH would put your CO2 at 4000ppm.
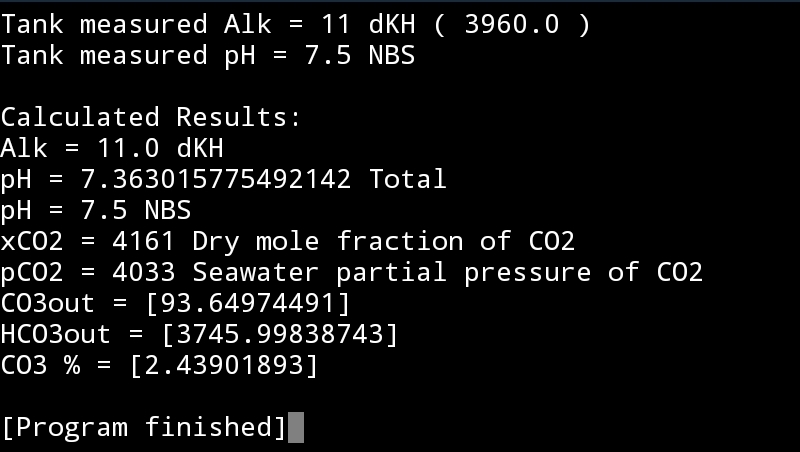
From a health perspective...
So if your feeling fine around your tank, chances are your home CO2 isn't near this level. So you have aeration or measurement issue.
A cup aeration test will help identify what's going on.
Can only report what I'm seeing. Nitrates when flow is slow and I don't tinker remain below 5 ppm and phosphates under 0.25 ppm. Test kits is API and I know they aren't accurate but I'm not looking for precission. Just absense or presence and extreme measurements such as post cycle nitrates of 160 ppm or showing zero. Wasn't curious as to exactly how high but that it was high. For all intents and purposes. Filtration is working and I've measured my DKH with both API and Tropic Marin and again not going to be precise but I'm looking to stay above 7 and below 11. At some point I'll get better test kits but for the moement this is all experimental and holistically seeking results.Also, if you dKH is stable (with no dosing as stated), the nitrogen cycle is not affecting dKH and hence not affecting pH.
If your NO3 rose by 50ppm your dKH would drop by 2.3dKH and the expected impact on pH would be as follows...keeping your CO2 levels at 4000.
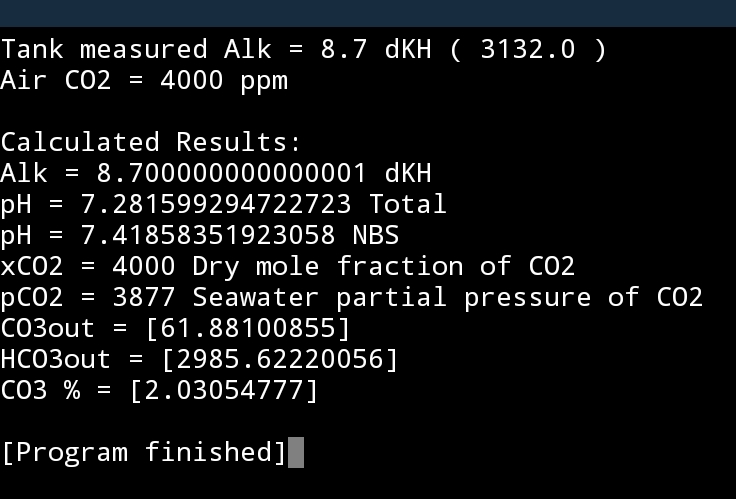
As you can see, this would only be a ~0.1 drop in pH.
House temps at 72, tank at 78 and outside currently at 54. It's literally impossible for me to perform the cup test. Best I just buy a home air quality measuring tool. I feel fine around my tank. Still getting and air exchanger at some point.
Can only report what I'm seeing. Nitrates when flow is slow and I don't tinker remain below 5 ppm and phosphates under 0.25 ppm. Test kits is API and I know they aren't accurate but I'm not looking for precission. Just absense or presence and extreme measurements such as post cycle nitrates of 160 ppm or showing zero. Wasn't curious as to exactly how high but that it was high. For all intents and purposes. Filtration is working and I've measured my DKH with both API and Tropic Marin and again not going to be precise but I'm looking to stay above 7 and below 11. At some point I'll get better test kits but for the moement this is all experimental and holistically seeking results.
Wasn't aware of that but at this point does it matter? My alk is at 11 and my PH at 7.9. Rather wait to test PH after a few days of returning to a slower flow as well as test all required parameters, again. If PH remained at 7.9 or basically above 7.8 then I'm golden because my main problem remains that I don't have consumers of alkalinity and was waiting until the PH stabilized above 7.8 so I can introduce coralline (added on Sunday) and corals which will come post establishment of coralline confirming system can support hard corals unless I get impatient which might likely happen. I also need to upgrade my light. That experiment failed miserably.A drop to from 25c to 12c will result in a pH increase of ~0.03. This is not significant for what the cup aeration test will identify...so you can go for the simple cup aeration test.
I think this is getting lost in translation. I no longer have a PH problem. Did at one time. Trying to better understand why. I posed a solution on aeration for which Randy provided information on why that might not be the case. At this point the conversation got off topic but it seems I'm learning and perhaps others might. Ecological systems being complex often have many variables affecting why something happens and better if we understand these variables and perhaps easier to understand and fix what went wrong. Hence me trying to understand why my PH was at 7.5. Wasn't because my measuring tool was wrong. That tool used is very accurate and was calibrated. Plus I used two different kinds of test strips to get a general confirmation. Again, not expected to be precision accurate but can show PH is lower than desired or somewhere in the vicinity of expected.I don't think your pH issue is due to the tank cycling nor the Reborn substrate as its just effectively coral skeleton.
You either have an aeration problem (not enough flow in the tank) or a measurement problem.
Wasn't aware of that but at this point does it matter? My alk is at 11 and my PH at 7.9. Rather wait to test PH after a few days of returning to a slower flow as well as test all required parameters, again. If PH remained at 7.9 or basically above 7.8 then I'm golden because my main problem remains that I don't have consumers of alkalinity and was waiting until the PH stabilized above 7.8 so I can introduce coralline (added on Sunday) and corals which will come post establishment of coralline confirming system can support hard corals unless I get impatient which might likely happen. I also need to upgrade my light. That experiment failed miserably.
I'm only human but try my best not to be.
I've been trying to grow coralline since the mid 80s. LOL. My current light is a Kessil Tuna Sun set to full spectrum. I'm confident it will grow corals considering it's very intense and at full power should provide enough of the blues corals evolved to use at their depths. However, at the required intensity I'm guessing provides the best PAR my rocks have become covered in green algae. Not GHA. Not slime. Just the kind of coating an African cichlid tank would die for. Introduced an urchin on Sunday and got to mowing that lawn but seems to be more content on my gravel since that top layer is Reborn and lots of green growing there. Might need to turn the gravel and make it white again although currently going through a blackout to hopefully lower the algae but need to be careful because I don't want lose the coralline. Never ends. LOLCompletely agree!
Just a note on coralline algae. It took my tank over 8 months before I really got good coralline growth...and I seeded it a couple different ways.
Hi hope all is well,if predictive tx predicts to many words incorrectly then can turn it off.Carbonate yes
I would like a phone that does not correct my wording or tells me how to spell lol

